Artigo de Pesquisa
In vitro inhibition of Escherichia coli from women with urinary tract infection by cranberry hydroalcoholic extract
Abstract
The antimicrobial potential of cranberry hydro alcoholic extracts (CrE) was evaluated against Escherichia coli isolated from women with urinary tract infection (UTI). CrE was diluted based on the percentage of proanthocyanidins (PACs) in extract powder for final concentrations: 1.26%; 2.52%; 3.35%, 5.03% and 10.06%. CrE antimicrobial potential was evaluated by disk and well diffusion assays, and by in vitro direct action against E. coli. Antibacterial action was observed for all performed tests: the minimal inhibitory concentration (MIC) was 1.26% PACs per disk diffusion assay and 2.52% of PACs by well diffusion assay. The in vitro antimicrobial direct action against E. coli resulted 3.8 Log10 cycles reduction for a concentration of 5.03% of PACs. One of the isolates showed multi resistance to antibiotics. but it was also inhibited more than any of the antibiotic tested in well diffusion assay. Only for concentrations 1.26%, 2.52% and 3.45% the inhibition of Escherichia coli by cranberry extract was dose-dependent, i.e directly proportional to the concentration of PACs. The results indicate a high potential for inhibitory action of CrE. However, more in vitro and in vivo analysis can be performed to fix which the best concentration of CrE capable of causing a real beneficial effect on UTI´s.
- Keywords:
- UTI.
- Proanthocyanidins.
- Pharmaceutical Plant.
- Vaccinium macrocarpon.
Introduction
It is estimated that around 150 million urinary tract infections (UTIs) occur worldwide each year. The average is 8 million cases in the United States alone. Urinary infection is the most common bacterial infection among adult women and active sex life[1] reaching between 50% and 60% throughout their lives[2].
E. coli stands out as the main etiological agent, with a percentage of occurrence of 75 to 95% of the clinical isolates of UTIs[3,4]. In women the recurrence of infection is common, mainly due to the anatomy of the urethra, in addition to other factors associated with the use of contraceptives, menopause [4], diarrhea or antifungals[5]. According to Strasinger & Lorenzo[6], UTIs are of different proportions, and can be characterized as: cystitis, a bacterial infection with inflammation in the ascending part of the bladder or lower urinary tract; an acute pyelonephritis or even a chronic pyelonephritis, causing renal structures abnormalities. UTIs are considered to be recurrent when two or more infections occur within a period of six or more than three months in a period of one year after curing of first infection[7,8].
In the United States of America, 11% of women over 18 years of age will have a bladder infection per year. Cystitis tends to be recur, unlike pyelonephritis and other types of UTIs[9]. Repeated treatment is also associated with increased resistance mechanisms against β-Lactams and fluoroquinolones[10].
The fruit cranberry (Vaccinium macrocarpon L) is a native fruit of North America, with records of cultivation around 1816. The fruit was described by North American Indians who used it for the treatment of UTIs[4,11]. Cranberry fruit has water, organic acids, fructose, vitamin C, flavonoids, anthocyanins, catechins, triterpenes, glycosides, anthocyanidins and proanthocyanidins (PACs). PACs are tannins that inhibit bacterial adhesion, constituting a system of natural defense of the plant[4,11].
Adherence to uroepithelium is the first step for the bacterium to develop an infection and this ability is determined by the electrical charge of the surface of the microorganism, hydrophobicity, fimbriae and adhesins[11-13]. The adhesins bind the exposed lectins to the host cells, where they adhere, multiply and cause infection[3,4,11]. Research indicates that the beneficial effect of cranberry on UTIs[14] occurs by blocking adhesion of E. coli to uroepithelium and therefore inhibit urinary infection[15]. Without bacterial adhesion, colonization does not occur in urinary tract[4,11].
In general, when a patient presents a probable UTI, the bacterium has already ascended the urethra towards the bladder, colonizing, multiplying and causing inflammation and severe pain[16]. Although indicators point to a decrease in E. coli adhesion after eight hours of cranberry intake, studies are still lacking to prove its effectiveness, metabolism, excretion, and protective action against UTIs [4]. It is possible that cranberry extract works as a coadjuvant in the prevention or treatment of diseases, especially related to UTIs [17,18]. However, the efficacy of prophylaxis with cranberry has not yet been confirmed. Several studies have shown that urinary tract infections are a public health problem, as well as being a frequent infection, especially in women, it can become repetitive as well as the use of antibiotics. In addition, hospitals are increasingly crowded, compromising treatment against bacterial resistance.
In view of the above, this study evaluated the antimicrobial potential of cranberry hydroalcoholic extract on E. coli strains get from women with urinary infection and residents in a rural community of Santa Catarina state, Brazil.
Materials and methods
A total of 2.550 medical records obtained of a rural community in the state of Santa Catarina, Brazil were used for this study. The research project was previously submitted to the Committee of Ethics in Research with Human Beings (Brazil Plataform), under protocol no. 49529615.5.0000.5367 and received a substantiated opinion of approval of no. 1.556,652.
Bacterial isolates and biochemistry confirmation
Bacterial clinical isolates nitrate positive and suggestive of Escherichia coli were obtained of urine. For each bacterial isolate, the Bactray system (Laborclin, São Paulo) was used for the biochemical identification of Gram-negative bacilli, oxidase-negative and glucose assimilation profile. Previously, Methylene Blue and Eosine agar (EMB, HiMedia Laboratories Pvt., India) was used for all typical E. coli isolates. The cultures were then maintained in standard Brain Heart Infusion agar (BHI, Difco, Brazil) for antimicrobial activity testing.
Preparation of Cranberry Extract
We used the dried extract of the fruit cranberry (Vaccinium macrocarpon L.) (Fagron, Holland) in the form of fine powder, colour reddish brown, with characteristic odor. The physics, chemistry and microbiological quality control data were evaluated according to the Manufacturing lot (code CMYJ20150102). The extraction solvent was ethanol/water (1:1) with estimated initial concentration of 25.16% proanthocyanidins.
Five different concentrations of the hydroalcoholic extract (ethyl alcohol PA: H20, 1: 1) of cranberry were prepared from stock solution corresponding to following percentage of proanthocyanidins (1:1): (25.16%), 1: 2.5: (10.06%), 1: 5: (5.03%), 1: 7.5: (3.35%), 1: 10: (2.52%) and 1: 20: (1.26%). The extracts were sterilized by membrane filtration (Millipore™, pore Ф 0.45 μm). These concentrations were determined based on the total percentage of proanthocyanidins contained in the sample. Therefore, the objective was to determine the lowest concentration capable of inhibiting E. coli.
Bacterial culture and tests
For each test performed, the bacterial isolates were reactivated in BHI broth for 18-24 hours. Culture aliquots were inoculated into BHI agar (Difco, Brazil) for isolating colonies after incubation at 35°C/24 hours. When necessary, BHI broth cultures of 18 h/35°C were also obtained for using liquid culture in log phase of growth. An inoculum with 1.5x106 CFU.mL-1 were used for disk or well diffusion tests.
Antimicrobial susceptibility by Escherichia coli isolates
The antimicrobial susceptibility profile (antibiogram) of clinical E. coli isolates was evaluated based on the disc diffusion technique according to the BrCAST (Brazilian Committee on antimicrobial susceptibility testing[18]. Were used: THE antibiotics (Cefar, São Paulo): Amikacin (30 μg), Amoxicillin/clavulanic acid (20 μg), Ampicillin (10 μg), Cephalotin (30 μg), Cefepime (30 μg), Ceftriaxone 30μg), Ciprofloxacin (5 μg), Gentamicin (10 μg), Piperacillin/azobactam (100 μg) and Trimethoprim/Sulfamethoxol 25/23.75 μg).
Antimicrobial activity
The indirect antimicrobial activity of cranberry extracts was evaluated based on well diffusion and disk diffusion assays:
Well diffusion (WD) assay[19] - cultures previously grown from each of the E. coli isolates in BHI broth were inoculated in plate (20 μL) in sterile Petri dish, adding 20 mL of 1% tryptone agar (Difco, Brazil). After agar solidification, the wells were perforated in agar and adding 35 μL of the respective dilutions of cranberry the hydroalcoholic extract - CrE (1:1, 1:20, 1:10, 1:7,5, 1:5 or 1:2.5). The antibiotics Cefotaxime (30 μg/mL) and Ceftazidime (30 μg/mL) were used as positive controls; negative controls consisted of: sterile water, ethanol PA and hydroalcoholic solution (1:1). The agar plates were incubated at 35/18-24 hours.
Disk diffusion (DD) – all clinical isolates of E. coli were previously developed on BHI agar (18-24 hours 37°C) for use in disk diffusion inhibition tests. The tests were realized according to the CLSI methodology (Clinical and Laboratory Standards Institute) using Mueller Hinton agar[19]. Sterile disks of 6.33 mm in diameter (Cefar, São Paulo) were individually impregnated with respective dilutions of CrE (35 μL per disk) well as all control groups, according previous test by well diffusion. Plates from each test were incubated at 35°C/18-24 hours. All tests were realized in triplicates with one repetition. The average value (mm of inhibition halos) obtained by the replicates of each test (WD or DD) against isolates of E. coli were added to compose the final mean, representing the action of extracts on E. coli cells.
Direct in vitro antimicrobial activity (DAA) of cranberry extract (CrE) on E. coli isolates
DAA of CrE against E. coli isolates was evaluated in 1% Buffered Peptone Water-BPW (Merck, Germany) to avoid interference of components of the culture medium on E. coli cell. The strain E. coli ATCC 25922 was used as standard control. Colonies grown for 18 hours in BPW for each one of E. coli isolates were diluted in 0.85% sterile saline solution. Spectrophotometer reading (Vis, λ 546nm) was used for adjustment to final concentration of 107 to 108 CFU/mL in each test. This count was previously determined by using 20 μL of aliquots of each culture grown and inoculated in blood agar plates (Neogen, Lansing, Michigan) to determine the number of colonies after incubation at 35°C/24 hours. Sterile 96-well cell culture plates were used for the tests. From each sample diluted in BPW broth (Merk, Germany) (pH 6.8) the following test groups were defined: A) cultures of nine E. coli isolates (1:10) in cranberry extract previously diluted in BPW broth 1: 5 (5.03% of PACs), concentration chosen according to the commercial indication used of ECr in capsules; B) control groups: I) E. coli isolate in BPW (1:10); II) sterile culture broth; positive controls: culture of E. coli ATCC 25922 at the same conditions as group A. All groups were incubated under orbital shaking (110 revolutions/minute) at 35-37°C in shaker (New Ethics Incubator 430/120 minutes. All the tests were performed in triplicate and with one repetition (3x2). After incubation, aliquots from each well were withdrawn and inoculated into blood agar plates (1.5%) in triplicates and incubated at 35°C/18-24 hours. The reduction or inhibition of colony growth obtained in each test was defined as CFU/mL of viable colonies recovered, i.e, compared to the control groups (without extract). The results were analyzed based on mean and standard deviation and Anova tests (Excel, Microsoft®) were performed to verify the occurrence of statistical difference (t-Test, p <0.05).
Results and Discussion
Clinical records of recurrent urinary tract infections (UTIs)
Were analysed 2.550 medical records of data obtaining during 4 years. Of these, 799 urine cultures were performed. It was found that 66 female patients (all age groups) had Urinary tract infection (UTI) in that period. Of these, 51 women were of childbearing age. Several bacterial species were present in different percentages. E. coli was present in 62.9% of these urinary infections and, on average, 3 infections occurred for each patient over four years (TABLE 1). Enterococcus sp. presented the second highest percentage (11.2%), appearing in 16 of the 143 infections that 51 women had within 4 years.
| Age | Number of women |
| 15 - 25 | 5 |
| 26- 35 | 8 |
| 36 -45 | 4 |
| 46 -55 | 6 |
| 56 - 65 | 9 |
| 66 - 75 | 9 |
| 76 - 88 | 10 |
| Total | 51 |
Identification of bacterial isolates
From the data of the year 2015 were evaluated n=860 urine reports which had positive indication of urinary tract infection. From these isolates, n=186 culture samples for E. coli isolates 186 were obtained. For the research n=10 bacterial isolates were available for the study. Each isolate was re-confirmed for E. coli by microbiological and biochemical identification (TABLE 2). Nine isolates were confirmed as E. coli and one isolate as Hafnia sp., but it was excluded of the present study. Considering the percentage of similarity from the biochemical tests performed, the E. coli isolates differed in the determination of the biochemical profile. The minimum percentage of specie similarity obtained with E. coli isolates for was 73.61% (one isolate) and two isolates showed the maximum, i.e. 100% of similarity for E. coli. Finally, from 9 strains, seven were determined as E. coli Type 1, and two as E. coli Type 2.
| E. coli* | (%) Identification / Type |
| 1 | 96.28 (E. coli Type 1) |
| 2 | 98.04 (E. coli Type 2) |
| 3 | 100.00 (E. coli Type 1) |
| 4 | 99.93 (E. coli Type 1) |
| 5 | 96.85 (E. coli Type 2) |
| 6 | 100.00 (E. coli Type 1) |
| 7 | 99.06 (E. coli Type 1) |
| 8 | 98.51 (E. coli Type 1) |
| 9 | 73.61 (E. coli Type 1) |
| Legend: * From typical colonies on EMB-Eosin Methylene Blue agar; ** Bactray Web I, II (Laborclin, São Paulo, Brazil). | |
Antimicrobial susceptibility
Since E. coli are increasingly involved in mechanisms of resistance against β-lactams and fluoroquinolones[20], in this study, the antibiotic susceptibility profile was evaluated for each of the nine strains of E. coli (TABLE 3).
| Antibiotics | Escherichia coli | |||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ATCC 25592 | |
| Amikacin (30μg) | S | S | S | S | I | S | S | S | S | S |
| Amoxicillin/Clavulanic Acid (20/10μg) | S | I | S | S | R | S | S | R | S | R |
| Ampicillin (10μg) | S | R | S | S | R | R | S | R | S | R |
| Cephalothin (30μg) | S | R | S | I | R | S | S | R | I | R |
| Cefepime (30μg) | S | S | S | S | R | S | S | S | S | S |
| Ceftriaxone (30μg) | S | S | S | S | R | S | S | S | S | S |
| Ciprofloxacin (5μg) | S | S | S | S | R | R | S | S | S | S |
| Gentamicin (10μg) | S | S | S | S | I | S | S | S | S | S |
| Piperacillin / Tazobactam (100/10 μg) | S | S | S | S | I | S | S | S | S | S |
| S = sensitive; R = resistant; I = intermediate. | ||||||||||
Five strains of E. coli had similar profiles of susceptibility to antibiotics, with only two of them exhibiting intermediate resistance to Cephalotin (β-lactam 1st generation). Two other presented resistance to three antibiotics, and one of them to two of the antibiotics, and finally one with resistance to six antibiotics used (E. coli isolate no. 5) and intermediate resistance to three antibiotics. Considering the mult resistance of the isolate no.5, it was evaluated how this isolate behaved against the action of hydroalcoholic extracts of cranberry containing different concentrations of proanthocynidins (PACs). The TABLE 4 shows the values obtained in well and disk diffusion assays, comparing them to E. coli ATCC 25922. It can be observed that by well diffusion, at the concentrations of 25.16% and 10.06% the isolate no. 5, a multidrug resistant, had similar data in relation to the reference lineage at least for three concentrations of PACs.
| Proanthocyanidins (PACs) in cranberry hydroalcoholic extract/mean values of inhibition diameter halos (ɸ, mm) in agar diffusion assay | |||||||
| Escherichia coli | |||||||
| (isolate no.5) | PACs (%): | 25.16 | 10.06 | 5.03 | 3.35 | 2.52 | 1.26 |
| WD | 12.33a | 12.33a | 12.00a | 9.67b | 4.33c | 2.33d | |
| DD | 9.00b | 8.33b | 4,67c | 0e | 0e | 0e | |
| E. coli | |||||||
| ATCC 25922 | WD | 12.33a | 12.00a | 10.67b | 9.33b | 8.00b | 4.00c, |
| DD | 7.67f | 6.33f | 4.33c | 3.33c | 0e | 0e | |
| Legend: Equal lowercase letters in lines and colunms indicate no statistical difference. | |||||||
Antimicrobial activity by well and disk diffusion tests by using hydroalcoholic extract of cranberry
The isolates were submitted to well diffusion and disk diffusion tests with the six concentrations of PACs from each sample of cranberry extract. The inhibition obtained by diffusion into wells was directly proportional to the concentration of each sample extract (FIGURE 1).
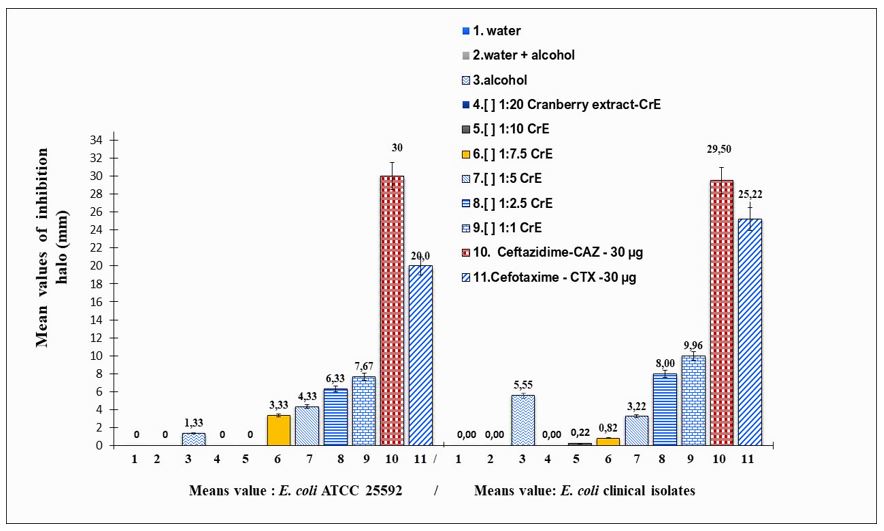
Direct in vitro antimicrobial activity of cranberry hydroalcoholic extract
The direct in vitro inhibition tests against microbial cells can provide more real data on the inhibitory potential of substances, since quantification of the population resulting from the action of the test substance on the untreated group (TABLE 5).
| E. coli | CFU.mL-1 initial count | CFU.mL-1 after cranberry extract in vitro action - PACs concentration (%): 5.03 | Reduction/ |
| Inhibition | |||
| (Log10 cycles) | |||
| 1 | 0.2 x 107 | 0.4 x 106 | 1 |
| 2 | 0.1 x 108 | 0.5 x 102 | 6 |
| 3 | 0.3 x 107 | 0 | 7 |
| 4 | 0.7 x 107 | 0.3 x 105 | 2 |
| 5 | 0.1 x 108 | 0 | 8 |
| 6 | 0.4 x 107 | 0.4 x 106 | 1 |
| 7 | 0.8 x 107 | 0.5 x 105 | 2 |
| 8 | 0.6 x 107 | 0 | 7 |
| 9 | 0.2 x 108 | 0.8 x 105 | 3 |
| 10. E. coli ATCC 25922 | 0.7 x 107 | 0.1 x 106 | 1 |
E. coli has been confirmed as the main etiologic agent of UTI (occurrence of 75 to 95%)[3,4]. We verify about 62.9% of UTI of female patients. This shows that in that rural municipality of the Midwest of Santa Catarina, UTIs are more frequent in female patients. The population of this municipality had, in 2010, around 8,700 inhabitants, with almost 50% of women. This means that about 1.17% of women have frequent urinary tract infections.
In general, studies related to cranberry evaluate bacterial inhibition using E. coli Type 1 and its fimbria-P. This is a protein filament that allows adhesion to the bladder epithelium of the host[4]. Even though E. coli type 1 is most implicated in urinary tract infections, other species have also been isolated, such as Proteus sp., Pseudomonas aeruginosa, Enterococcus faecalis, Staphylococcus aureus, such as here in which was isolated a strains of Hafnia sp.[9,20-24].
Urinary tract infections are a big problem, not just by frequency but also by becoming recurrent or repetitive. Recurrent urinary tract infections (UTIr) are characterized by the occurrence of two or more episodes in six months, or three or more episodes per year after the first UTI has been cured[8]. A study conducted in the USA demonstrated the increase in bacterial resistance of E. coli to some antibiotics over a 10-year period. The bacteria acquired an increase of 14.1% for Ciprofloxacin and for Trimethoprim / Sulfamethoxazole an increase of 6.3%. Other antibiotics did not increase significantly, such as Amoxicillin/Clavulanic acid (increase of 0.3%) and Nitrofurantoin (increase of 0.8%). In this study, two bacteria were resistant to Ciprofloxacin and two to Trimethoprim/Sulfamethoxazole.
In our study the isolate no. 5 of E. coli was resistant at least six antibiotics. Thus, it can be considered as multidrug resistant, and a concern for women with UTI´s. The data indicated a direct action of the CrE in culture which was able to eliminate the population in relation to the control group after 2 hours. Therefore, CrE was efficient in eliminating the multiresistant strain.
The indiscriminate use of antimicrobials is worrying. But it does not rule out the need of developing new drugs for prevention, treatment or prophylaxis of these UTIs[20]. Informally, cranberry extract has been indicated as a prophylactic agent related to UTIs. The activity of bacterial inhibition in vitro has been evaluated based on the ability of adhesion to host cells. Thus, Lavigne et al.[22] analyzed bacterial anti-adhesion activity in vitro using urine from healthy patients experimentally contaminated with different strains of E. coli. In that study, there was an in vitro anti-adhesion effect with urine samples from patients treated with three 108 mg/day (group 1) capsules compared to the placebo group (group 2). Study realized by using the concentrations of PACs of 0.64, 128 and 345.8 mg. mL-1 were used in the co-culture of E. coli with human uroepithelial cells. Thus, it was observed in vitro inhibition of bacterial adhesion[23]. The antimicrobial direct action of the CrE (dilution 1: 2.5) corresponding to the dose of cranberry extract marketed in capsules (approximately 400 mg/capsule) showed elimination of the population after the treatments. However, about 5.03% of PACs (approx. 200 mg/capsule) also was used and it was considered more adequate to evaluate the real power of direct in vitro inhibition of the extract on E. coli. Furthermore, it has a better cost-benefit ratio. As a result, there was a total reduction in the number of colonies for two of the strains and inhibition of the others in relation to the initial concentration of microorganisms of each control group.
The resistance to antimicrobials derived from plants is considered low[24,25]. This drives studies on the antimicrobial potential of plant extracts[26,27]. Côté et al.[25] evaluated the antimicrobial effect of different extracts of cranberry on gram positive and gram negative bacterial pathogens. They observed an inhibitory rather than bactericidal effect.
At the present study the mean of inhibition halo was 12.33 mm for 25.16% of PACs by using cranberry extract in well diffusion assay. It is known that the action of tannins on bacterial membranes occurs mainly by the chelation of metallic ions[21]. Cranberry is rich in condensed tannins (PACs)[3,4,11] and possibly the mechanism of inhibition of cranberry is the same, since the PACs are condensed tannins[4,11].
Here, there was no inhibition difference between the concentrations of 25.16% and 10.06% of PACs, and it occurred even at the lowest concentration, i.e., 1.26% of PACs. Cranberry extract was more inhibitory than the antibiotics Ceftazidime and Cefotaxime, used as controls (positive). This indicates a great inhibition potential.
Only for the concentrations 1.26%, 2.52% and 3.45% of PACs the inhibition of Escherichia coli by cranberry extract was dose-dependent, i.e directly proportional to the concentration of PACs. Cranberry extract was more efficient at eliminating one of the multi-resistant antibiotic isolates than any of the antibiotic tested. The results indicate a great inhibitory action potential of the cranberry extract. However, additional in vitro e also in vivo analysis may be performed to determine the best concentration of cranberry extract capable of having a real beneficial effect on UTI´s. In addition, studies of the mechanisms of action of PACs on E. coli can help to understand better the process of bacterial adhesion in urinary infections.
Funding
This study had financial support from National Council for Scientific and Technological Development, and Government of Santa Catarina State, Brazil (2015-2017).
Acknowledgements
To all women directly or indirectly involved in this study. We hope that the research can contribute to the issues related to bacterial resistance and, mainly, the use of natural antimicrobial compounds for the treatment of urinary infections.
Referências
1. Karlowsky JA, Lagacé-Wiens PR, Simner PJ, DeCorby MR, Adam HJ, Walkty A, et al, Antimicrobial resistance in urinary tract pathogens in Canada from 2007 to 2009: CANWARD surveillance study. Antimicrob Agents Chemother. Jul. 2011; 55(7): 3169-75. [CrossRef] [PubMed].
2. Nelius T, Winter C, Willingham J, Filleur S. Immune-Based Treatment Strategies for Patients with Recurrent Urinary Tract Infections – Where Are We? In: Nelius T, ed. [on- line]. Recent Advances in the Field of Urinary Tract Infections. Texas: In Tech. 2013; p.1-15. ISBN 978953511180. Acesso em: 05 nov. 2018. Disponível em: [Link].
3. Hancock V, Ferrières L, Klemm P. Biofilm formation by asymptomatic and virulent urinary tract infectious Escherichia coli strains. FEMS Microbiol Lett. Feb. 2007; 267(1): 30-7. [CrossRef] [PubMed].
4. Hisano M, Bruschini H, Nicodemo AC, Srougi, M. Cranberries and lower urinary tract infection prevention. Clinics. 2012; 67(6): 661-7. ISSN 1807-5932. [CrossRef].
5. Zhong YH, Fang Y, Zhou JZ, Tang Y, Gong SM, Ding XQ. Effectiveness and safety of patient initiated single-dose versus continuous low-dose antibiotic prophylaxis for recurrent urinary tract infections in postmenopausal women: a randomized controlled study. J Int Med Res. 2011; 39(6): 2335-43. ISSN 1473-2300. [CrossRef] [PubMed].
6. Strasinger SK, Lorenzo MSD. Urinálise e fluídos corporais. 5ª ed. São Paulo: LMP, 2009. 329p. ISBN 9788599305324.
7. Foxman B. Recurring urinary tract infection: incidence and risk factors. Am J Public Health. 1990; 80(3): 331-3. [CrossRef] [PubMed].
8. Drekonja DM, Johnson JR. Urinary tract infections. Primary care. 2008; 35(2): 345–67. [CrossRef] [PubMed].
9. Pallett A, Hand K. Complicated urinary tract infections: practical solutions for the treatment of multiresistant Gram-negative bacteria. J Antimicrob Chemother. 2010; 65 (Suppl 3): iii25-33. [CrossRef] [PubMed].
10. Rice LB. Mechanisms of resistance and clinical relevance of resistance to β-Lactams, glycopeptides, and fluoroquinolones. Mayo Clinic Proceedings. 2012; 87(2): 198-208. [CrossRef] [PubMed].
11. Foo LY, Lu Y, Howell AB, Vorsa N. The structure of cranberry proanthocyanidins which inhibit adherence of uropathogenic P-fimbriated Escherichia coli in vitro. Phytochemistry.2000; 54(2): 173-81. [CrossRef] [PubMed].
12. Jepson RG, Williams G, Craig J. Cranberry for preventing urinary tract infections 2013; 131(5): 363. ISSN: 1516-3180. [CrossRef].
13. Uberos J, Iswaldi I, Carretero R, Fernadez-Puentes V, Molina-Carballo A, Muñoz-Hoyos A. Cranberry (Vaccinium macrocarpon) changes the surface hydrophobicity and biofilm formation of E. coli. Microbiol. Insights. 2011; 4: 21–27. ISSN: 11786361. [CrossRef].
14. Guay DRP. Cranberry and urinary tract infections. Drugs. 2009; 69(7):775–807. [CrossRef] [PubMed].
15. Pérez-López FR, Haya J, Chedraui P. Vaccinium macrocarpon: An interesting option for women with recurrent urinary tract infections and other health benefits. J Obst Gynaecol Res. Aug. 2009; 35(4): 630–639. [CrossRef] [PubMed].
16. Howell AB. Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res. Jun. 2007; 51(6): 732-7. [CrossRef] [PubMed].
17. Jepson RG, Craig JC. A systematic review of the evidence for cranberries and blueberries in UTI prevention. Mol Nutr Food Res. Jun. 2007; 51(6): 738-45. [CrossRef] [PubMed].
18. Brazilian Committee on antimicrobial susceptibility testing - BrCAST - Método de Disco-Difusão para Teste de Sensibilidade aos Antimicrobianos. 2017, version 6. Disponível em: [Link]. Acesso em: 05 mai. 2018.
19. Valgas C, Souza SM, Smânia EFA, Smânia Jr. A. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007; 38(2): 369-380. ISSN 1517-8382. [CrossRef].
20. Sanchez GV, Babiker A, Master RN, Luu T, Mathur A, et al. J. Antibiotic resistance among urinary isolates from female outpatients in the United States in2003 and 2012. Antimicrob Agents Chemother. 2016; 60(5): 2680-3. [CrossRef] [PubMed].
21. Maia-Araújo YLF, Mendonça LS, Orellana SC, Araujo ED. Comparação entre duas técnicas utilizadas no teste de sensibilidade antibacteriana do extrato hidroalcoólico de própolis vermelha. Sci Plena. 2011; 7(4): 1-4. Disponível em: [Link] [acesso em: 18 mai. 2018].
22. Lavigne JP, Bourg G, Combescure C, Botto H, Sotto A. In-vitro and in-vivo evidence of dose-dependent decrease of uropathogenic Escherichia coli virulence after consumption of commercial Vaccinium macrocarpon (cranberry) capsules. Clin Microbiol Infect. 2008; 14(4): 350-5. ISSN 0956-7135. [CrossRef] [PubMed].
23. Pinzón-Arango PA, LIU, Camesano TA. Role of cranberry on bacterial adhesion forces and implications for Escherichia coli-uroepithelial cell attachment. J Med Food.Apr.2009; 12(2): 259-70. [CrossRef] [PubMed].
24. Funatogawa K, Hayashi S, Shimomura H, Yoshida T, Hatano T, Ito H, et al. Antibacterial activity of hydrolyzable tannins derived from medicinal plants against Helicobacter pylori. Microbiol Immunol. 2004; 48(4): 251-61. [CrossRef] [PubMed].
25. Côté J, Caillet S, Doyon G, Dussault D, Sylvain J-F, Lacroix M. Antimicrobial effect of cranberry juice and extracts. Food Control.2011; 22(8): 1413-1418. [CrossRef] [CrossRef].
26. Akiyama H, Fujii K, Yamasaki O, Oono T, Iwatsuki K. Antibacterial action of several tannins against Staphylococcus aureus. J Antimicrobial Chemoth. Oct. 2001; 48(4): 487-491. [CrossRef] [PubMed].
27. Ohno T, Kita M, Yamaoka Y, Imamura S, Yamamoto T, Mitsufuji S, et al. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter. Jun. 2003; 8(3): 207-15. [CrossRef] [PubMed].