Artigo de Pesquisa
Comparison of the effects of aqueous extract of Sida cordifolia L. and 5-fluorouracil in colon carcinogenesis induced by 1,2- dimethylhydrazine on Wistar rats
Abstract
Search for new therapeutic agents and alternative strategies for chemoprevention of colorectal cancer is needed to reduce morbidity and mortality resulting from this disease. This study aimed to compare the effect of aqueous extract of Sida cordifolia L. and 5- fluorouracil (5-FU) on colon carcinogenesis induced by 1,2- dimethylhydrazine (1,2-DMH). The extract reduced the frequency of aberrant crypts regarding to the positive control, but there was no significant difference among the positive control animals, those who received the extract and those receiving standard anticancer drug. Thus, the aqueous extract of Sida cordifolia L. 800 mg/kg showed no statistical impact on carcinogenesis in animal models, but showed a significant anti-inflammatory effect on the colon mucosa.
- Keywords:
- Colorectal cancer.
- Chemoprevention.
- Sida cordifolia L.
- Aberrant crypt.
- 1,2-dymethylhydrazine.
Introduction
Colorectal cancer (CRC) is a health problem that affects people all over the globe. It has an annual incidence of approximately 1 million cases and over 500,000 fatal cases per year[1]. The risk of colorectal cancer varies considerably from one country to another, and even within a country. Its highest rates occur in the most developed continents[1,2]. Perceptible differences in its incidence around the world led to the hypothesis that these variations could be explained in general by environmental influences, related to changes in lifestyles such as high consumption of animal fat, as well as the influence of genetic factors[2]. In Brazil, according to the report from Instituto Nacional do Câncer (INCA), there were an estimative of 41,010 new cases of colorectal cancer in 2020, of which 20,540 in men and 20,470 in women[3]. In 2019, data from Sistema de Informação de Mortalidade (SIM) revealed 20,578 fatal cases[4]. About 70% of colon cancer patients are over 65 years of age and the disease is rare for ages under 45[5].
Sida cordifolia L. is a medicinal herb widely used in Indian Ayurvedic medicine to treat diseases such as asthma, chronic dysentery and gonorrhea[6]. In its phytochemistry, there are compounds such as asparagine, tryptophan, alkaloids (ephedrine – sympathomimetics, hypaphoforine, vasicine, vasicinone, vasicinol – bronchodilator), phytosterols, mucin, rutin and potassium nitrate[7,8]. Analgesic, anti-bacterial, anti-inflammatory, hypoglycemic and hepatoprotective effects have also been reported in several studies[9,10]. In Brazil, it is used mainly as a natural anti-inflamatory[11].
Studies on anti-tumor effects are still scarce, although there have been positive results. Sida cordifolia L. is a component of PADMA-28, a Tibetan herbal preparation used as a T cells antiproliferative agent in lymphoblastic leukemia. PADMA-28 seems to have an effect in apoptosis induction, blocking cells in G1 phase and fragmenting poly-ADP-ribose-polymerase[12]. One of the constituents of Sida cordifolia L. (cryptolepine, an alkaloid) was identified in a screening on p21-inducing compounds (a tumor suppressor gene). Cryptolepine was isolated and used in osteosarcoma and colon cells, wich were wild and knockout for p21. After evaluation the antiproliferative effect of the substance in these two conditions, a decrease of 47% and 12%, respectively, was observed in cell viability, which revealed the potential of the derivative of Sida cordifolia L.[13].
The purpose of this study is to compare the antiproliferative action of 5-fluorouracil (5-FU) with an herbal extract of Sida cordifolia L. on colon carcinogenesis in rats. It is a search for an alternative treatment or chemoprevention for colorectal cancer.
Material and Methods
The species of Sida cordifolia L. (white mallow) were collected in the city of Lagarto – Sergipe, in a thorp called Sobrado (close to the coordinates -10,95929; -37,62183), and were identified as ASE 36.306 in the herbarium of the Federal University of Sergipe. The study was approved by Animals Research Ethics Comittee of the Federal University of Alagoas(CEUA/UFAL), in August 2014, with registration 024/2014.
Preparation of the aqueous extract of Sida cordifolia L.
We collected a sample of 4,5 kg of white mallow. The material was exposed to the sun for drying for 15 days. After drying, it was mashed without detaching the plant structures. The whole processed material was extracted by placing 12 L of heated distilled water and filtered through filter paper sheets. We shared the solution in fractional volumes of 250 mL into 7 glass vials. Lyophilization at 3000 rotations per minute was performed intermittently, starting at 8 am and ending at 7 pm daily for 3 weeks. The solid residues were scraped from the freeze-drying vials, obtaining a yield of 112 g of aqueous extract. The extract was diluted in distilled water to reach a solution of 120 mg/mL. It was administred to rats at a dosage of 800 mg/kg, via gavage, as a chemoprevention protocol.
Experiment protocol
Fifteen 5 to 6-week old Wistar rats were tested, weighing between 200 and 240 g. They were divided into three groups distributed in three boxes and treated for 13 weeks (FIGURE 1). The 3 groups were: Box I (5 rats receiving induction and treatment); Box II (5 rats receiving only induction and placebo); Box III (5 rats receiving chemoprevention and induction). All subjects were submitted to a 12h/12h light/dark cycle and were fed with standard food and water ad libitum[14,15].
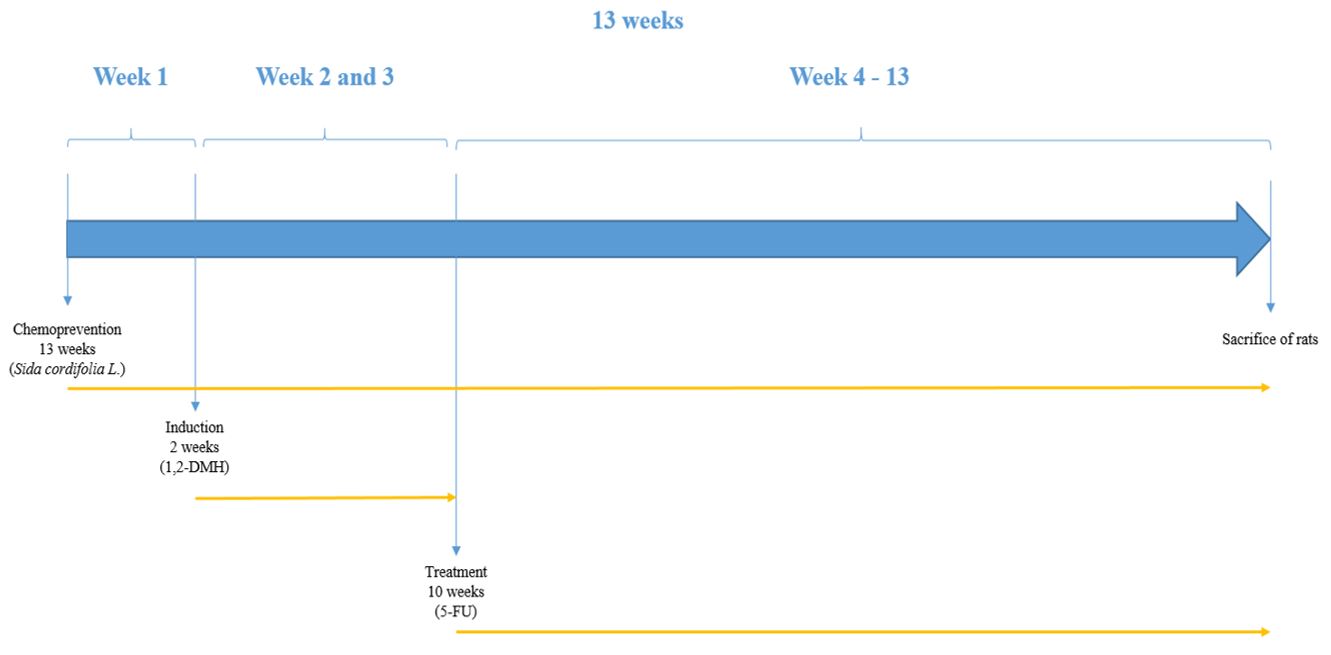
.Techniques used in the study: chemoprevention - administration of 800 mg/kg of the aqueous extract of lyophilized Sida cordifolia L.,via gavage; induction - administration of 4 doses of 1,2- dimethylhydrazine, intraperitoneally, 2 doses per week; treatment – weekly intraperitoneal administration of 50 mg/kg of 5-fluorouracil.
The induction of aberrant crypt foci (ACF) in the colonic mucosa was achieved by the administration of 30 mg/kg of 1,2-DMH e intraperitoneally twice a week for 2 weeks[16]. After 13 weeks of experimentation, the animals in the 3 groups were sacrificed. The colon was removed by laparotomy with a median xiphopubic incision, opened at anti-mesocolic border and washed with saline solution. The samples were stored in tubes with 10% buffered formalin solution. The specimen was sent to anatomopathological analysis for identification and quantification of ACFs.
Statistical analysis
The results were subjected to statistical analysis of variance (ANOVA) and Tukey's test using SPSS software (version 15.0).
Results and Discussion
Number of aberrant crypt foci per group
Frequency of aberrant crypt foci per colon segment
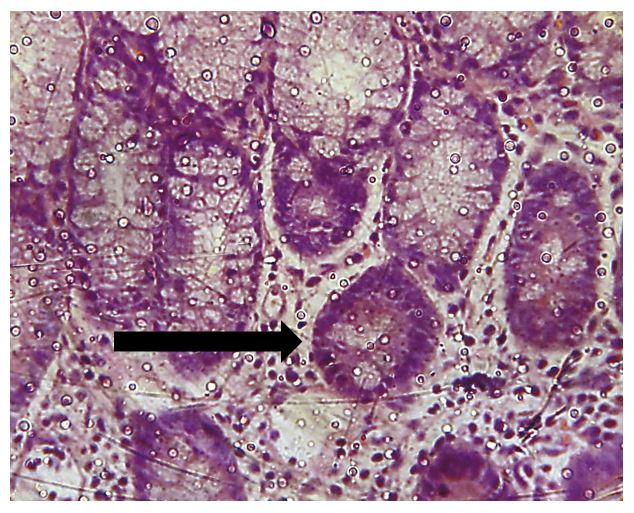
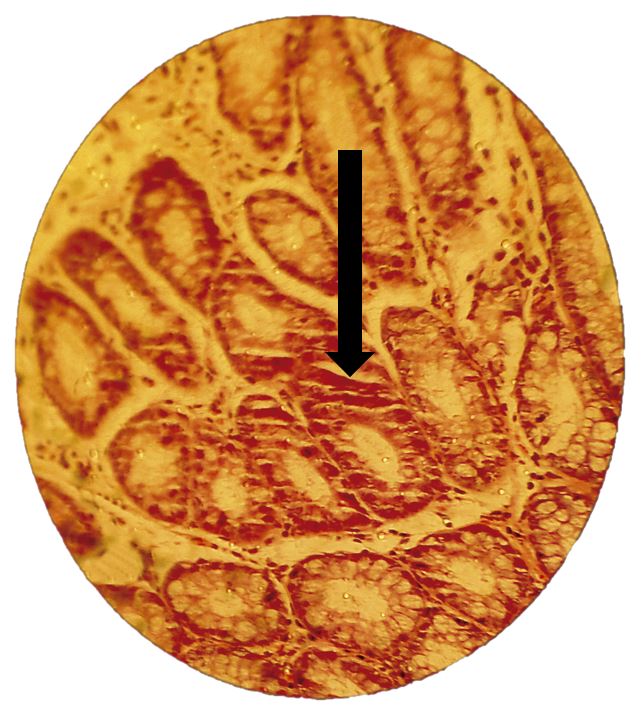
Aberrant crypts are initial pre-malignant lesions of the colon mucosa, which can be induced in the large intestine of rodents by chemical carcinogenesis[17]. 1,2-DMH is a potent carcinogenic for the formation of dysplasia and even neoplasia, depending on exposure time according to previous studies[18]. These are lesions present in Kirsten rat sarcoma oncogene (KRAS) and adenomatous polyposis coli (APC) mutations, altering the structure of the crypt. They can be atypical/hyperplastic, in cases in wich there is an increase in the number of cells per aberrant crypt compared to typical glands, and dysplastic in variyng degrees, when they present hypochromic, pleomorphic and elongated nuclei with loss of polarity, stratified or pseudostratified epithelium, lumen dilation to 1,5 times the typical glands and loss of the differentiation of caliciform globet cells[17,19-21].
All 15 animals survived the total duration of the experiment. Histological analysis demonstrated a greater amount of aberrant crypt foci in the distal segment compared to the proximal segment, representing approximately 75% of the lesions found (FIGURES 2, 3 and 4). The amount of aberrant crypt was greater in the distal segment of the colonic mucosa of rats in comparison to the proximal segment, as shown by studies with Kenaf and Gynura extracts[14,22]. This lesion distribution is also compatible with data that showing that 70% of colon tumors in humans are distally located in rectum and sigmoid regions[23].
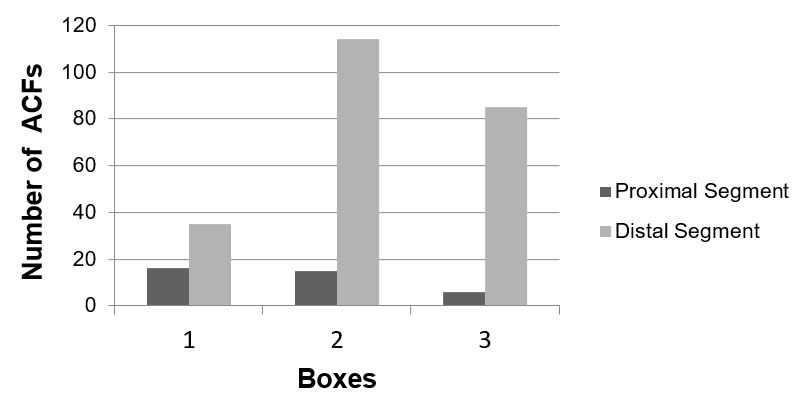
There was a reduction in the absolute total amount of colonic crypt in the groups treated with 5-FU (Box I - 69%) and with the extract of Sida cordifolia L. (Box III - 25%) compared to the positive control group (Box II) (TABLE 1). The 69% reduction of crypt incidence in Box I compared to Box II (p=0,056) suggests the well-known antiproliferative effect of 5-FU as a drug used in chemotherapy treatment for colorectal cancer. The 25% reduction of crypts in the Box III (treated with the extract) was not associated with the significant variation of results in relation to the positive control group (Box II; p=0.26). Thus, although there were positive results with the extract in vitro, regarding the proliferation of neoplastic cells[24,25], this was not verified in vivo with the 800 mg/kg dosage used in this study's methodology.
| Proximal | Distal | Total | |
| G I | 16 | 35 | 51 |
| G II | 15 | 114 | 129 |
| G III | 6 | 85 | 91 |
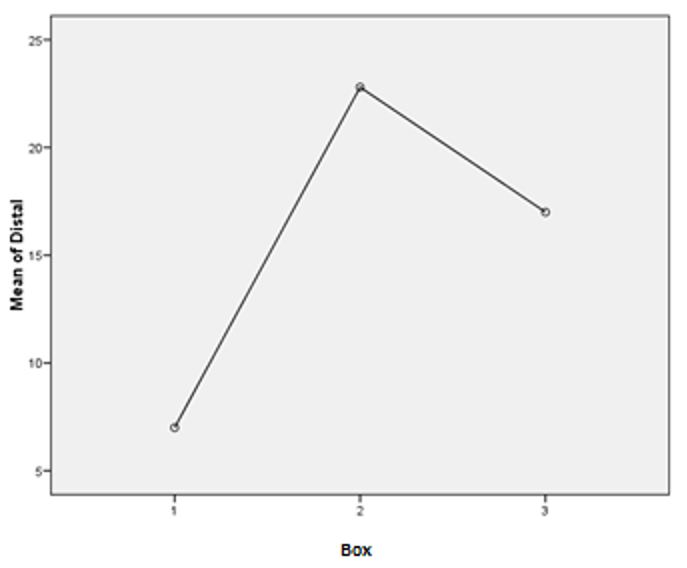
Comparison of the number of ACFs among the groups
Statistical analysis of the distal segments of the subjects in all 3 boxes have shown results close to the 95% confidence interval (p=0.06). The graph below (FIGURE 5) depicts this segment revealing a greater number of lesions in the positive control group compared to the other groups. A lower incidence of crypts was observed in the group treated with 5- FU compared to the groups that received aqueous extract of Sida cordifolia L. For the other groups, data on the number of ACFs was not sufficient to make a statistically significant comparative analysis.
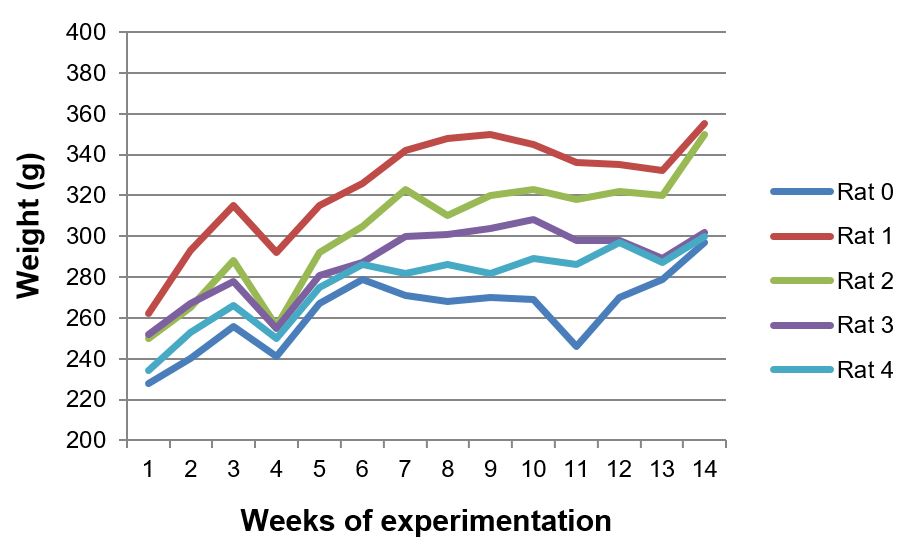
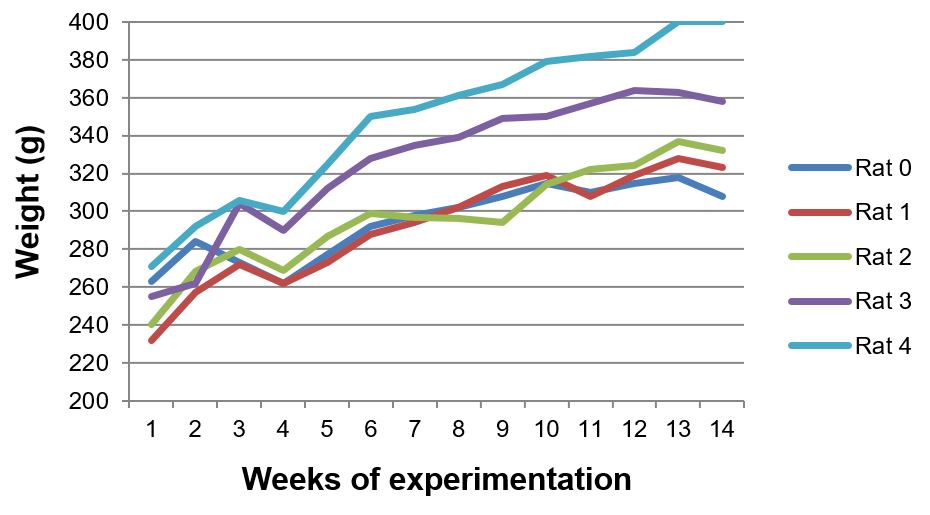
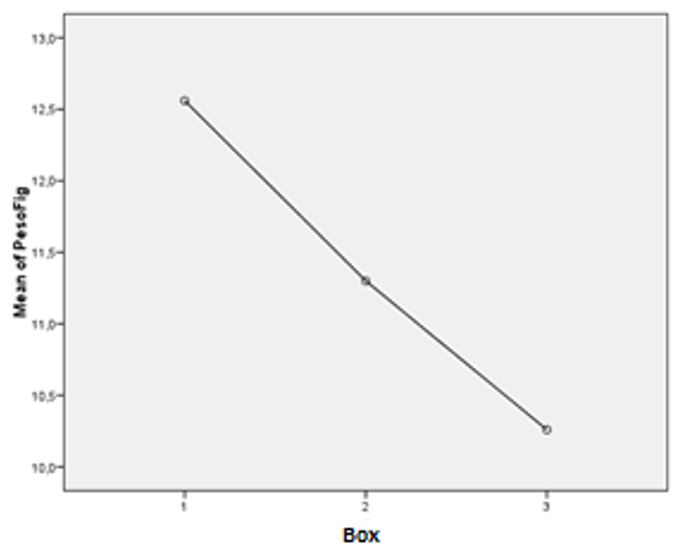
Body and liver weight of animals
The body weight of all animals increased during the 13 weeks of experimentation. The rats treated with the aqueous extract had a steadier growth during the time of exposure (FIGURES 6, 7 and 8). Less weight variation and a more regular growth rate throughout 13 weeks in group III can be related to metabolic effects associated with the extract of Sida cordifolia L., such as hypoglycemia and stimulated release of insulin by pancreatic beta cells[9].

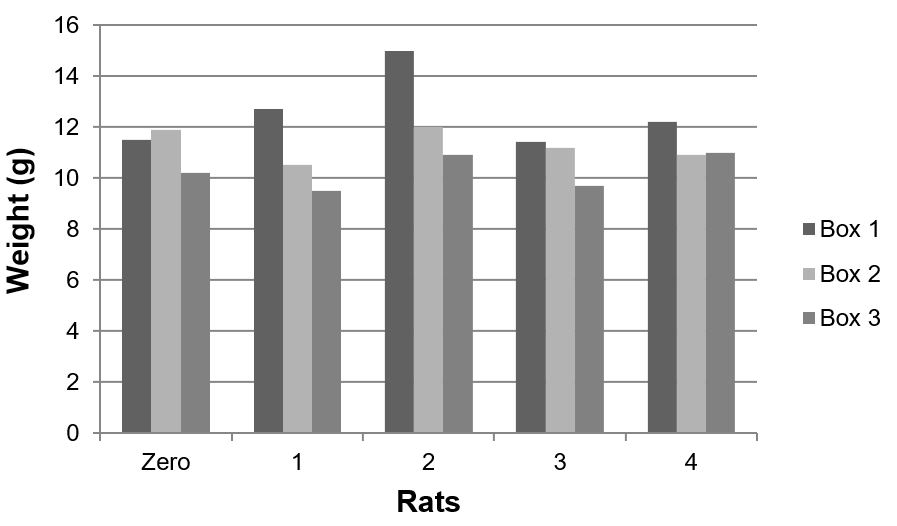
The three groups differed significantly in terms of liver weight (p=0,012). The liver weight of the animals treated with aqueous extract of Sida cordifolia L. was lower than those that received only 1,2-DMH associated with 5-FU (p=0,009) (FIGURE 9 and 10). Silva et al.[11] demonstrated a dose-dependent liver regeneration after hepatectomy when administrated alcoholic extract of Sida cordifolia L., revealing the species' hepatoprotection characteristic. Mallikarjuna et al.[26] demonstrated a reduction in serum levels of transaminases with increasing doses of aqueous extract of Sida cordifolia L. Such an effect may be suggested in our study by this reduction of hepatic weight among the subjects of group III compared to groups I and II, with a significant difference between the animals that received the extract and those that received the 5-FU-associated carcinogen (p=0,009).
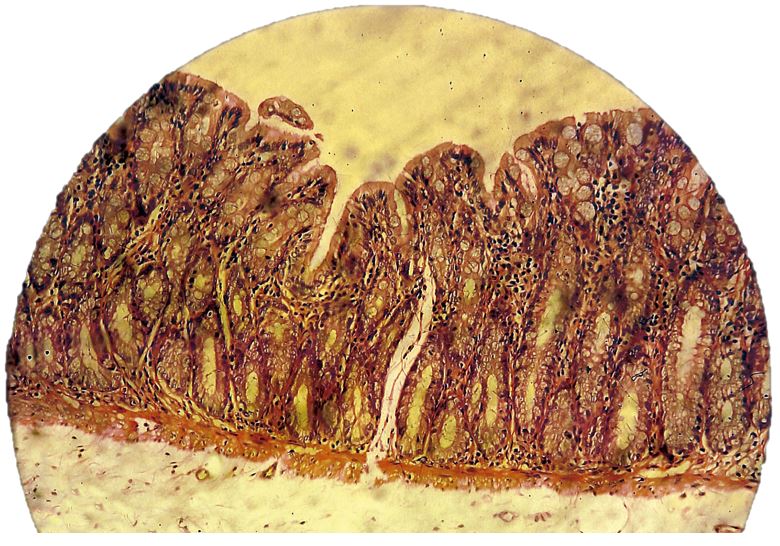
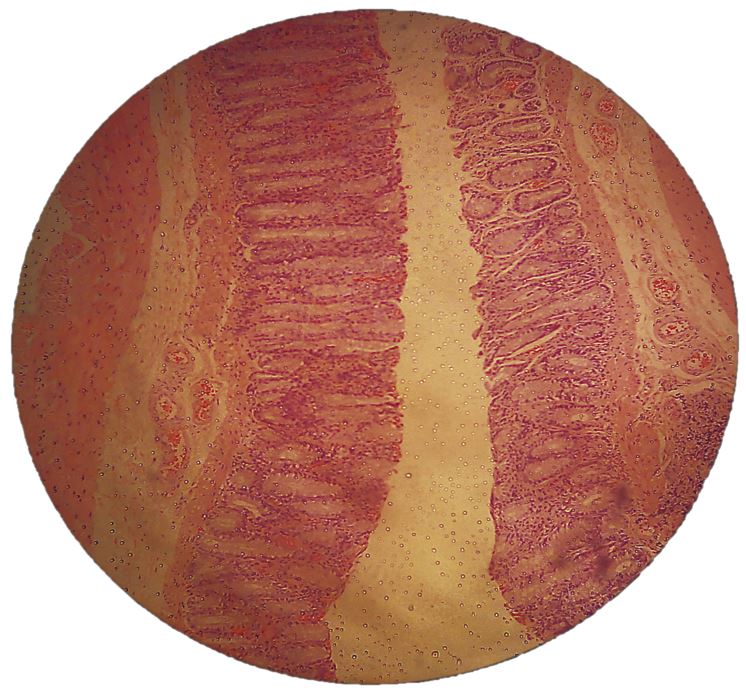
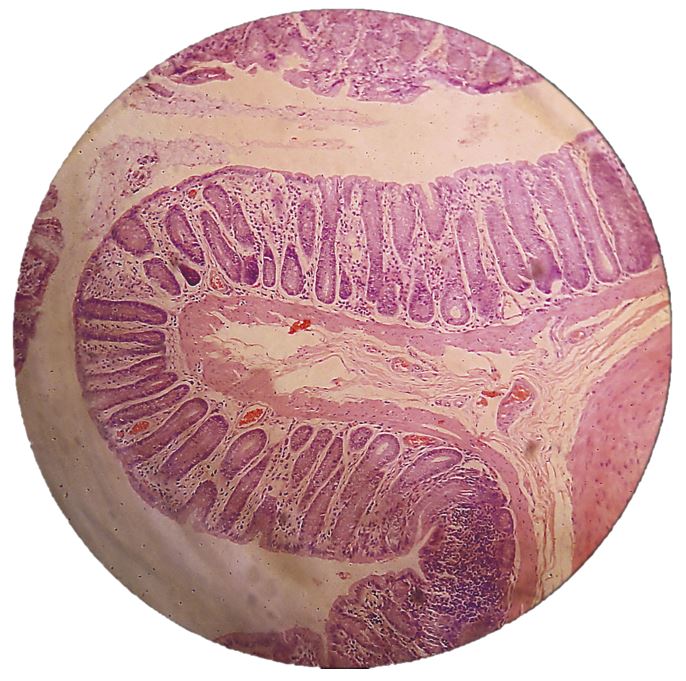
Colonic mucosa inflammation
The colon mucosa of the subjects in all three groups receiving 1,2-DMH presented an infiltration of inflammatory cells, with lymphoid clusters, fibroblast proliferation and reactive lymph nodes. The histological findings of the subjects treated with aqueous extract were much less infiltrated by inflammatory cells and presented less proliferation of fibroblasts (FIGURES 11, 12 and 13). The anti-inflammatory effect of the extract of Sida cordifolia L. is described in many previous studies[6,9,11] involving paw edema[9,27,28] and an evaluation of the reduction of free radicals[26], as well as its contractile effect on the vascular system by alpha-adrenergic stimulation[29]. The presence of specific alkaloids and ephedrine is related to this bioactivity. This study showed a reduction of inflammatory activity in the colitis induced by the carcinogen 1,2-DMH administered intraperitoneally, given the noticeable decrease in inflammatory cell infiltration in the colon mucosa of subjects of group III in comparison to groups II and I, along with a minor proliferation of fibroblasts also in the group that received the aqueous extract of Sida cordifolia L. by gavage.
Conclusion
Although one of the elements of the aqueous extract of Sida cordifolia L. – cryptolepine - reduces the amount of neoplastic clones in osteosarcoma and colon cells in vitro, and the evidence of its anticancer effect on hepatomas[30], in our study 800mg/kg of aqueous extract did not significantly reduce the number of aberrant crypts in the colon mucosa of rats with lesions induced by the administration of 1,2-DMH when compared to the positive control group. On the other hand, the results of the study have strengthened the evidence on hepatoprotection due to a significant decrease in liver weight compared to another experimental group, as well as its natural anti-inflammatory effect on carcinogen-induced colitis.
Acknowledgments
We would like to thank the professors PhD Emiliano Barreto and Salete Smaniotto for making the Celular Biology Laboratory available for this study, and to the undergraduate and PhD candidates at Celular Biology Laboratory and Natural Resources Research Laboratory, who provided us with technical support in their respective laboratories. We would also like to thank Maria Auxiliadora Andrade, who collected samples of Sida cordifolia L. in loco.
References
1. World Gastroenterology Organisation, International Digestive Cancer Alliance Practice Guidelines. Colon cancer screening [Internet]. 2007. 18p [Cited 2015 Ago 12] Available from: [https://www.worldgastroenterology.org/UserFiles/file/guidelines/colorectal-cancer-screening-english-2007.pdf].
2. Suzuki R, Kohno H, Sugie S, Tanaka T. Sequential observations on the occurrence of preneoplastic and neoplastic lesions in mouse colon treated with azoxymethane and dextran sodium sulfate. Cancer Sci. 2004; 95(9): 721–27. [https://doi.org/10.1111/j.1349-7006.2004.tb03252.x] [https://pubmed.ncbi.nlm.nih.gov/15471557/].
3. Brasil. Instituto Nacional do Câncer. Estimativa dos casos novos [Internet]; 2020 [Cited 01 Apr. 2021]. Available from: [https://www.inca.gov.br/estimativa/estado-capital/brasil].
4. Brasil. Ministério da Saúde. DATASUS. Sistema de Vigilância em Saúde; Sistema de Informações sobre Mortalidade [Internet]. 2019 [Cited 01 Apr 2021]. Available from: [http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sim/cnv/obt10uf.def].
5. Labianca R, Nordlinger B, Beretta GD, Brouquet A, Cervantes A. Primary colon cancer: ESMO Clinical Practice Guidelines for diagnosis, adjuvant treatment and follow-up. Ann Oncol. 2010; 21(Suppl 5): 70-7. [https://doi.org/10.1093/annonc/mdq168] [https://pubmed.ncbi.nlm.nih.gov/20555107/].
6. Pattar PV, Jayaraj M. Pharmacognostic and phytochemical investigation of Sida cordifolia L.- a threatened medicinal herb. Int J Pharm Pharm Sci. 2012; 4(1): 114-17. ISSN 0975-1491. [https://innovareacademics.in/journal/ijpps/Vol4Issue1/2882.pdf].
7. Khurana N, Sharma N, Patil S, Gajbhiye A. Phyto-pharmacological properties of Sida cordifolia: a review of folklore use and pharmacological activities. Asian J Pharm Clin Res. 2016: 9(Suppl. 2): 52-8. ISSN 2455-3891. [https://doi.org/10.22159/ajpcr.2016.v9s2.13698].
8. Martins CA, Campos ML, Irioda AC, Stremel, DP,Trindade AC, Pontarolo R. Anti-Inflammatory effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens is related to inhibition of prostanoid production. Molecules. 2017; 22(1883): 1-15. [https://doi.org/10.3390/molecules2211188] [https://pubmed.ncbi.nlm.nih.gov/29099738/].
9. Jain A, Choubev S, Singour PK, Raiak H, Pawar RS.Sida cordifolia (Linn) – An overview. J Appl Pharma Sci. 2011; 1(2): 23-31. [https://pesquisa.bvsalud.org/portal/resource/pt/sea-150743].
10. Venkatachalam D, Sebastian AC, Anu VB, Mathew C, Leon D, Thomas J et al. Investigation of pharmacognostical and preliminary phytochemical characters of Sida cordifolia. Int J of Res Pharm Pharm Sci. 2019; 4(3): 35-9. ISSN 2455-698X. [https://www.researchgate.net/publication/333675820].
11. Silva RL, Melo GB, Melo VA, Antoniolli AR, Michellone PRT, Zucoloto S et al. Effect of the aqueous extract of Sida cordifolia on liver regeneration after partial hepatectomy. Acta Cir Bras. 2006; 21(1): 37-9. [https://doi.org/10.1590/S0102-86502006000700009].
12. Bams APS, Jenny M, Schwaiger W, Bernhard D, Wrulich OA, Fuchs D et al. Apoptosis induced by the Tibetan herbal remedy PADMA 28 in the T-cell- derived lymphocytic leukaemia cell line CEM-C7H2. J Carcinog. 2005; 4:15. [https://doi.org/10.1186/1477-3163-4-15] [https://pubmed.ncbi.nlm.nih.gov/16138918/].
13. Matsui T, Sowa Y, Murata H, Takagi K, Nakanishi R, Aoki S et al. The plant alkaloid cryptolepine induces p21WAF1/CIP1 and cell cycle arrest in a human osteosarcoma cell line. Int J Oncol. 2007; 31(4): 915-22. [https://pubmed.ncbi.nlm.nih.gov/17786325/].
14. Shwter AN, Abdullah NA, Alshawsh MA, Alsalahi A, Hajrezaei M,Almaqrami AA et al. Chemoprevention of colonic aberrant crypt foci by Gynura procubens in rats. J Ethnopharmacol. 2014; 151(3): 1194-201. [https://doi.org/10.1016/j.jep.2013.12.044] [https://pubmed.ncbi.nlm.nih.gov/24393787/].
15. Ismail M, Ghafar SA, Yazan LS, Tahir PM. Kenaf seed supercritical fluid extract reduces aberrant crypt foci formation in azoxymethane-induced rats. Exp Toxicol Pathol. 2012; 64(3): 247-51. [https://doi.org/10.1016/j.etp.2010.08.016] [https://pubmed.ncbi.nlm.nih.gov/20869858/].
16. Srimuangwong K, Tocharus C, Suksamrarn A, Chintana PY. Effects of hexahydrocurcumin in combination with 5-fluorouracil on dimethylhydrazine-induced colon cancer in rats. World J Gastroenterol. 2012; 18(47): 6951-59. [https://doi.oeg/10.3748/wjg.v18.i47.6951] [https://pubmed.ncbi.nlm.nih.gov/23322993/].
17. Papanikolau A, Wang QS, Papanikolau D, Whiteley HE, Rosenberg DW. Sequential and morphological analyses of aberrant crypt foci formation in mice of differing susceptibility to azoxymethane-induced colon carcinogenesis. Carcinogenesis. 2000; 21(8): 1567-72. [https://doi.org/10.1093/carcin/21.8.1567].
18. Juca MJ, Bandeira BC, Carvalho DS, Leal AT. Comparative study of 1,2-dimethylhydrazine and azoxymethane on the induction of colorectal cancer in rats. J Coloproctol. 2014; 34(3): 167- 73. [https://doi.org/10.1016/j.jcol.2014.06.003].
19. Fenoglio-Preiser CM, Noffsinger A. Aberrant crypt foci: a review. Toxicol Pathol. 1999; 27(6): 632- 42. [https://doi.org/10.1177/019262339902700604] [https://pubmed.ncbi.nlm.nih.gov/10588543/].
20. Bird R, Mcllean E. Aberrant Crypts: Potential preneoplastics lesions in the murine colon. Cancer Res. 1988; 48(21): 6187-92. [https://pubmed.ncbi.nlm.nih.gov/3167865/].
21. Takayama T, Katsuki S, Takahashi Y, Ohi M, Nojiri S, Sakamaki S et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. NEJM. 1998; 339(18): 1278-84. [https://doi.org/10.1056/NEJM199810293391803] [https://pubmed.ncbi.nlm.nih.gov/9791143/] .
22. Ghafar SAA, Yazan LS, Tahir PM, Ismail M. Kenaf seed supercritical fluid extract reduces aberrant crypt foci formation in azoxymethane-induced rats. Exp Toxicol Pathol. 2012; 64: 247-51. [https://doi.org/10.1016/j.etp.2010.08.016].
23. Herfindal ET, Helms RA, Quan DJ. Textbook of therapeutics: drug and disease management. 8th ed. Philadelphia: Lippincot Williams & Wilkins; 2006.
24. Galal A, Raman V, Khan IA. Sida cordifolia, a traditional herb in modern perspective – a review. Curr Tradit Med. 2015; 1(1): 5-17. [https://doi.org/10.2174/2215083801666141226215639]
25. Buthkar PM, Suganthi V, Buthkar MV, Kothai R. In vitro antiproliferative activity of ethanolic extract of Sida cordifolia Linn against various cancer cell lines. Al Ameen J Med Sci. 2020; 13(4): 234-41. ISSN 0974-1143. [https://www.cabdirect.org/globalhealth/abstract/20210006604]
26. Mallikarjuna G, Prabakharan V, Sree LK. Pharmacological activities of Sida cordifolia: a review. IJPT 2013; 4(5): 315-21.
27. Sutradhar RK, Ahmad M, Bachar SC, Saha A, Guha SK, Rahman AM. Bioactive alkaloid of Sida cordifolia Linn. with analgesic and anti-inflammatory activities. IJPT. 2006; 5 (2): 175-8. [https://www.researchgate.net/publication/43561262].
28. Franzotti EM, Santos CV, Rodrigues HM, Mourão RH, Andrade MR, Antoniolli AR. Anti-inflammatory, analgesic activity and acute toxicity of Sida cordifolia L. (Malva Branca). J Ethnopharm. 2000; 72(1-2): 273-78. [https://doi.org/10.1016/s0378-8741(00)00205-1][https://pubmed.ncbi.nlm.nih.gov/10967481/].
29. Santos MRV, Moreira IJA, Filho JMB, Medeiros IA. Contractile effect of hydroalcoholic extract of Sida cordifolia L. leaves in rat aorta. Scientia Plena. 2013; 9(12): 1-6. [https://www.scientiaplena.org.br/sp/article/view/1585].
30. Mallikarjuna G, Reddy VJS, Prabakharan V. Evaluation of anticancer activity of Sida cordifolia L. against aflatoxin B1 induced hepatocellular carcinoma. Int J Pharm Sci Rev Res. 2013; 23(2): 126-32. ISSN 0976 – 044X. [https://www.researchgate.net/publication/286221617].