ARTIGO DE PESQUISA
Brazilian essential oil of Cymbopogon martinii: positive effects on inflammation-induced human fibroblasts and skin aging
Abstract
This study evaluated the effects of essential oil from Cymbopogon martinii (CMEO) on lipopolysaccharide (LPS)-stimulated human fibroblast cells. Samples of CMEO were collected in Monte Verde, Minas Gerais, Brazil. The fibroblasts were cultured and stimulated by LPS (1 μg/mL), and incubated for 24 h at 37°C. The cytotoxicity of CMEO was evaluated by MTT assay and collagen concentration by Sirius red. Collagenase activity, hyaluronic acid, and the concentrations of IL-1β; IL-6; MCP-1 (CCL2), and MIP-1-α (CCL3) were evaluated by ELISA assay. The effect of CMEO on the expression of mRNA and secretion of MMP-1, MMP-2, and MMP-9 enzymes were evaluated by RT-qPCR and ELISA, respectively. CMEO was cytotoxic against fibroblasts, in which 10 μg/mL inhibited 50% of cell viability. When treated with CMEO, the fibroblasts produced more collagen and hyaluronic acid compared to control cells. When stimulated by LPS, fibroblasts exhibited higher production of IL-6, IL-1β, MCP-1, and MIP-1α compared to control cells. However, the treatment of fibroblasts with CMEO reduces cytokines secretion and enzyme expression. The study showed that CMEO modulates inflammation mediators and reduces metalloproteinase mRNA and secretion levels, making it a promising candidate for anti-aging and wound healing treatments.
- Keywords:
- Cymbopogon martini.
- Anti-inflammatory.
- Anti-aging.
- Hyaluronic acid.
- Collagen.
Introduction
Essential oils (EOs) (Aetherolea, Ethereal oils, Volatile oils) are concentrated hydrophobic liquids containing volatile aromatic compounds from the secondary metabolism of plants[1,2]. EOs are principally composed of mono- and sesquiterpene hydrocarbons and their oxygenated derivatives, along with aliphatic alcohols, aldehydes, and esters[3]. The knowledge of EOs chemical profile is important to select the suitable extraction method[3].
EOs can be extracted from plant tissues, such as flowers, fruits, leaves, peel, stem, and roots [1,2]. Although they are largely used in the pharmaceutical and food industry, it is unknown exactly whether EOs were used as healing agents or for domestic use at the beginning[3].
Cymbopogon martinii (CM), as known as Palmarosa, is a member of the Gramineae family which is very famous for its high oil conten[1]. Cymbopogon martinii essential oil (CMEO) has been widely used in aromatherapy because of its antimicrobial and anti-inflammatory properties[4]. The bioactivity of EOs depends on the interaction of their compounds which results in a synergic or an antagonic effect[3]. Geraniol (3,7-dimethylocta-trans-2,6-dien-1-ol) is an acyclic monoterpene alcohol[4]. This monoterpenoid is the major compound of Palmarosa EO and has antioxidant and anti-inflammatory effects[4-6].
Fibroblasts are one of the most important cells involved in tissue repair because they produce a group of molecules to maintain the extracellular matrix (ECM) and replace wounded tissue, such as collagen type I, III, and IV, fibronectin, glycosaminoglycans, hyaluronic acid, laminins, metalloproteinases and proteoglycans[7]. In addition, ECM reorganization occurs through a process of degradation and crosslinking enzymes, produced by fibroblasts, that are activated and regulated by pro-inflammatory cytokines[7].
Although inflammation is a process well-regulated, an excessive inflammatory response can be a source of additional damage to individuals, such as inflammatory diseases[8]. Thus, the use of Palmarosa EOs as an anti-inflammatory agent can be an effective strategy to regulate an excessive inflammatory response[8]. The aim of the present study was to evaluate the effects of CMEO on lipopolysaccharide-stimulated human fibroblast cells.
Material and Methods
Iscove's Modified Dulbecco's Medium (IMDM), fetal bovine serum (FBS), penicillin- streptomycin, and phosphate-buffered saline (PBS) were obtained from Gibco BRL (Grand Island, CA, USA). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, Mo., USA).
Plant identification
Samples of Cymbopogon martinii were collected in the Monte Verde, State of Minas Gerais, Brazil, September 2019. An exsiccate of the plant collected and identified was deposited and authenticated (voucher specimen number HUMC 7661) in the Herbarium of the University of Mogi das Cruzes, SP, Brazil.
Cymbopogon martinii essential oil
The Essential oil of Cymbopogon martinii was supplied by WNF Essential Oils (São Paulo, SP, Brazil). The purity and quality of the Cymbopogon martinii essential oil were monitored by the WNF Essential Oils quality control department. Cymbopogon martinii chemical analysis was performed in the Analytical Center of the Chemistry Institute of the University of São Paulo (USP), by gas chromatography-mass spectrometer (GC-MS) Shimadzu model QP5050A, according to operating conditions: CBP-5 capillary column (50 m × 0.25 mm × 0.25 μm), injector temperature of 250°C and helium (He) as a carrier gas. The energy of impact used in MS was 70 eV. Compounds identification in EO was carried out by mass spectra analysis according to the National Institute of Standards and Technology library. The relative concentration of each compound in essential oil was computed from GC peak areas by the analysis program.
Culture and cytotoxicity evaluation of CMEO by MTT assay
The CCD1072Sk cell line was obtained from Rio de Janeiro Cell Bank (CCD1072Sk - ATCC CRL2088). The cells were cultured in a monolayer using IMDM (Gibco) supplemented with 10% fetal bovine serum (FBS), 100 UI/mL penicillin/streptomycin, and 0.25 μg/mL Fungizone (Gibco) in a humidified atmosphere at 37°C in 5% CO2. These cells were trypsinized three times per week using 0.25% trypsin/EDTA (Cultilab, Brazil). For the 24-hour cell viability assessment, the control and treated cells were centrifuged and resuspended in equal parts medium and trypan blue (0.05% solution) and counted using a hemocytometer. To evaluate the cytotoxicity of CMEO, the essential oil was dissolved in the culture medium in appropriate concentrations. The cell viability of control and CMEO (0.0–160.0 μg/mL)-treated fibroblasts cells were measured using a standard MTT assay. Briefly, 5×104 viable cells were seeded into clear 96-well flat-bottom plates (Corning) in IMDM medium supplemented with 10% fetal bovine serum (FBS) and incubated with different concentrations of the extract for 24h. Then, 10 μL/well of MTT (5 mg/mL) was added and the cells were incubated for 4h. Following incubation, 100 μL of 10% sodium dodecyl sulfate (SDS) solution in deionized water was added to each well and left overnight. The absorbance was measured at 595 nm using a FlexStation 3 Multi-Mode Benchtop Reader (Molecular Devices, Sunnyvale, CA, USA).
Cell viability after LPS treatment
Briefly, cells were seeded in 96-well culture plates at a density of 5×104 viable cells/well and incubated for 24 h, then exposed to IC50 concentration of CMEO (10 μg/mL), previously determined in item 2.1 in the presence of LPS (1 μg/mL) and incubated for another 24 h at 37°C. The MTT solution was added to a final concentration of 0.5 mg/mL and then incubated for 2 h at 37°C followed by the addition of 0.1mL of dimethyl sulfoxide to dissolve the MTT-formazan. The amount of MTT-formazan was then determined by measuring abs at 595 nm.
Sirius Red Collagen Quantification
The cells were pre-treated in serum free medium for 6 hours and were then treated with 10 µg/mL (IC50 concentration) of CMEO for 24 hours. After cells were cultured, the medium was removed, and the wells were washed three times with 0.1 M PBS. Next, 100 μl of Bouin's solution (picric acid 0.9%, formaldehyde 9.0% and glacial acetic acid 5.0%) were added for fixation for 1 h. Samples were washed with PBS, then the Sirius Red dye was added. After 1 h, the maximum possible amount of dye was removed, followed by washing with 150 μl of a 0.01 M hydrochloric acid solution for 30 seconds to remove the dye that did not bind to collagen. Next, the dye was removed from cell layers by the addition of 0.1 M NaOH for 30 min. 100 μL aliquots of the solution contained in the wells were transferred to a new plate. Absorbance was measured with an Elx- 800-UV (Bio-Tek Instruments, USA) microplate reader at 570 nm.
Hyaluronic acid synthesis assay
Effects of CMEO on HA synthesis were determined using an ELISA kit. The fibroblast cells (5.0 × 104 cells/well) were seeded in 96-well-plates for 24 h at 37°C, 5% CO2. The cells were pre-treated in serum free medium for 6 hours and were then treated with 10 µg/mL (IC50 concentration) of CMEO for 48 hours. The cultured medium was collected, and HA synthesis was measured by ELISA. The absorbance was measured at 450 and 570 nm using a microplate reader. The HA concentration in the cultured supernatant acquired from the treated fibroblast cells was calculated and compared with the standard curve of HA.
Collagenase activity
The collagenase activity was analyzed by using enzyme-linked immunosorbent assay (ELISA) kits (ABCAM, USA) following the manufacturer's instructions. Briefly, the cells (5.0 × 104 cells/well) were seeded in 96-well-plates for 24 h at 37°C, 5% CO2. Fibroblast cells were pre-treated in serum free medium for 24 hours and were further treated with CMEO (10 µg/mL) for 48 hours.
Cytokines and chemokines analysis in cell culture supernatants
The concentrations of IL-1β; IL-6; MCP-1 (CCL2); and MIP-1-α (CCL3) in the cell culture supernatants were analyzed by using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems, Minneapolis, MN, USA) following the manufacturer's instructions. Cells were treated with LPS (1 μg/mL) with or without CMEO (10 μg/mL) for 6 h. The cell culture supernatant (100 μL) was collected to determinates the levels of cytokines and chemokines, according to the manufacturer's instructions.
Reverse transcription‑quantitative PCR (RT‑qPCR)
The effect of CMEO on the expression of mRNA of MMP-1; MMP-2; and MMP-9 enzymes in fibroblasts was evaluate by RT-qPCR. Cells were pretreated with CMEO (10 µg/mL) for 2 hours and treated with LPS (1 µg/mL) for 24 hours. Total RNA extracted from cells samples was converted to cDNA using a SuperScript® III RT kit (Invitrogen, Carlsbad, CA), according to the manufacturer's protocol. The concentration of RNA was detected using a NanoDrop 2000 (Thermo Fisher Scientific, Inc.). GAPDH was used as the internal control. The thermocycling conditions were as follows: 95˚C for 10 min followed by 36 cycles of 94˚C for 15 sec and 57˚C for 40 sec. The 2‑ΔΔCq method was used to quantify the relative gene expression levels of the target genes. Relative standard curves were generated by serial dilutions and all samples were run in triplicates. Following, the sequence of primers used in the qRT-PCR analysis: MMP-1: Forward 5'-AGCTAGCTCAGGATGACATTGATG-3'; and reverse 5'-GCCGATGGGCTGGACAG-3'; MMP-2: Forward 5′-ACCGCGACAAGAAGTATGGC-3; and Reverse 5′-CCACTTGCGGTCA TCATCGT-3´; MMP-9: Forward 5′-CGATGACGAGTTGTGGTCCC-3; and Reverse 5′-TCGTAGTTG GCCGTGGTACT-3; GAPDH: Forward 5´-CGGTGTGAACGGATTTGGC-3´; and Reverse 5´-GTGAGTGGAGTCATACTGGAAC-3´.
Enzyme-linked immunosorbent assay for the determination of MMP2 and MMP9
Cell supernatant was centrifuged at 12,000 g for 15 min at 4°C. MMP2 ELISA kits (RAB0365; Sigma, USA) and MMP9 ELISA kits (RAB0372; Sigma, Nanjing, China) were used to measure the levels of protein secretion of MMP2 and MMP9 according to the manufacturer's instructions. To detect MMP-1 concentration in supernatants, ELISA was performed with a commercially available ELISA kit (Abcam). The MMP-1 concentrations were detected according to the manufacturer's instructions.
Statistical analysis
The obtained results were expressed as the mean ± standard error of mean (SEM) from at least three independent experiments, unless stated otherwise. Paired data was evaluated by Student's t-test. A p value of <0.05 was considered significant.
Results and Discussion
The main chemical components obtained by gas chromatography are shown in TABLE 1.
| RT* | Chemical composition | Area % |
|---|---|---|
| 8.818 | Geraniol | 81.70 |
| 10.488 | Geranyl acetate | 10.21 |
| 6.525 | Linalool | 01.57 |
| 9.014 | Geranial | 01.23 |
| 11.185 | Isocaryophyllene | 01.09 |
| *Retention Time. | ||
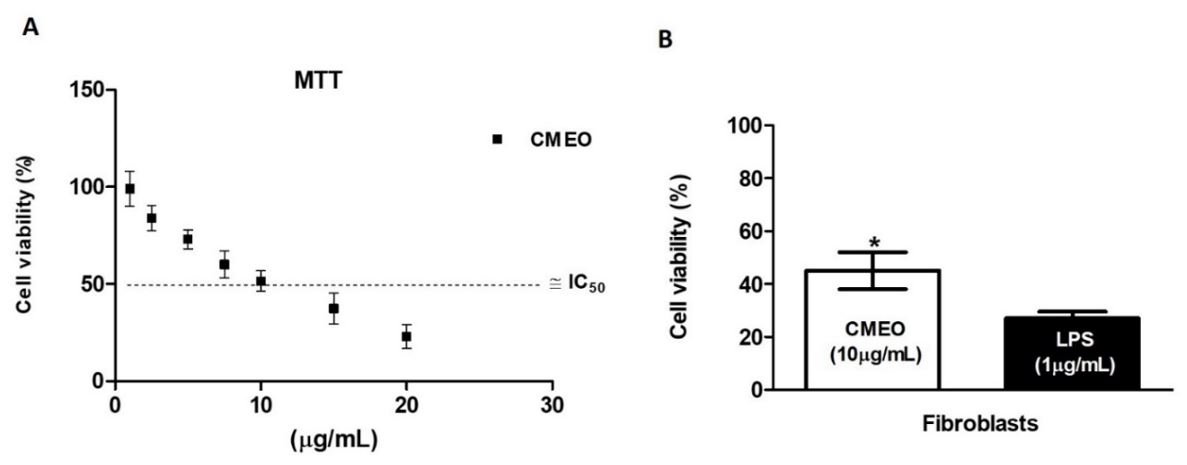
The effects of the CMEO on CCD1072Sk fibroblasts cell viability was evaluated using the MTT assay. As shown in FIGURE 1A, the CMEO was cytotoxic against fibroblasts cells, in which 10 μg/mL inhibited 50% of cell viability. The IC50% value obtained corroborates the variability found in the literature in studies where screenings are carried out with different types of fibroblasts and essential oils. CMEO was able to reverse the cytotoxicity caused by LPS in all these cell lines (FIGURE 1B).
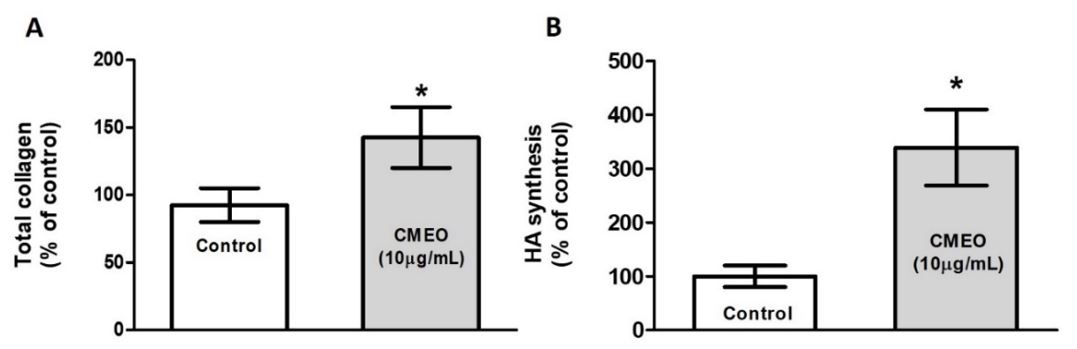
After the MTT assay and the determination of the IC50%, the subsequent aim was to investigate whether the CMEO was, in fact, able to increase collagen and hyaluronic acid synthesis. The FIGURE 2 show the effects of CMEO on collagen (FIGURE 2A) and hyaluronic acid (FIGURE 2B) synthesis. In both cases, the synthesis from CCD1072Sk fibroblast cells were found to have significantly increased when compared to control (non-treated cells).
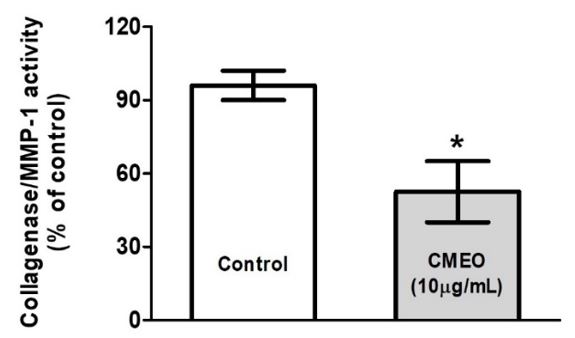
It is well known that collagenases are the enzymes that digest collagen. Therefore, the reduction of collagenase activity could protect against collagen breakdown. Thus, the effects of CMOE on collagenase activity showed that the essential oil dramatically decreased after treating the CCD1072Sk fibroblast cells (FIGURE 3).
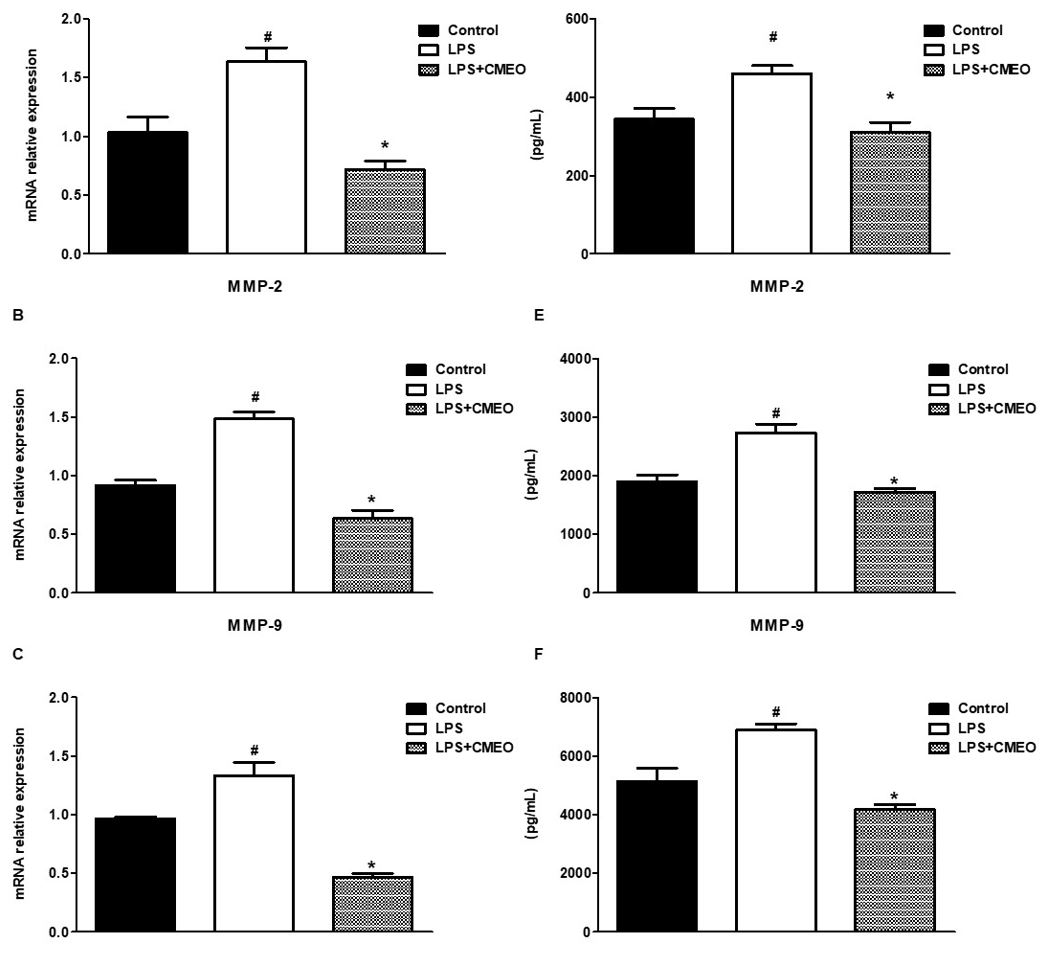
The FIGURE 4 (A, B and C) indicates that MMP-1 (collagenase), MMP-2, and MMP-9 mRNA enzymes were highly expressed in the LPS-activated fibroblasts cells. CMEO at 10 μg/mL significantly and similarly inhibited the mRNA expression of the metalloproteinases (MMP´s) enzymes as shown in the same FIGURE 4 (A, B and C). At the same time, the effects of CMEO (10µg/mL) and LPS on the levels of MMP-1, MMP-2, and MMP-9, were evaluated, as shown in FIGURES 4 (D, E and F) in conditioned medium from fibroblasts cultures.
The cytokines, IL-1β and IL-6, and the chemokines MIP-1α and MCP-1 were highly expressed in LPS-activated cells, however, the treatment with CMEO (10 μg/mL) significantly inhibited the expression of these inflammatory mediators in activated CCD1072Sk cells (FIGURE 5). The CMEO used in this study showed a high geraniol content (TABLE 1).
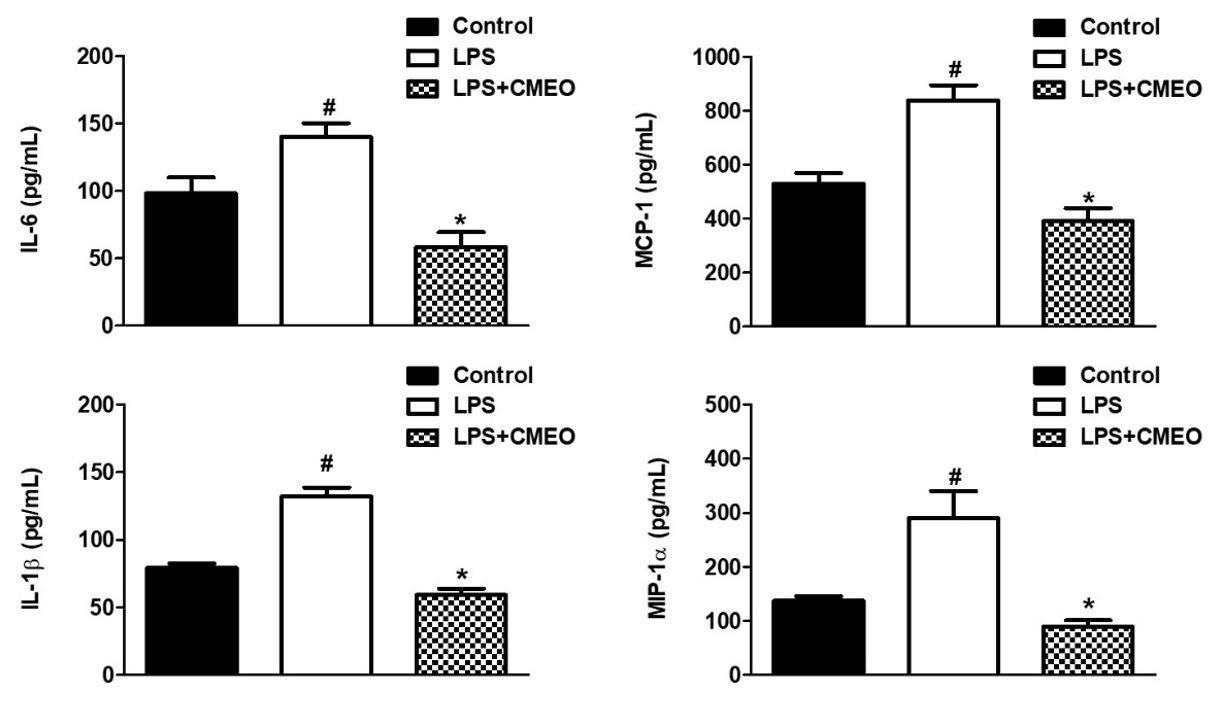
The major component found in CMEO was geraniol, corroborating data from the literature that indicate this as the main component of the essential oil of palmarosa (81,70%)[2,10,11]. In a screening carried out with different essential oils and V79 fibroblasts, it presented IC50% values between 19.50±5.96 and 448.00±19.52 μg/mL[11]. In a study with L929 fibroblasts tested with Moringa oleifera essential oil, it presented an IC50% of 42.99 ± 0.17 μg/mL[12].
An important biological factor associated with skin aging is the general atrophy of the extracellular matrix, reflected by a reduction in collagen levels[13]. In addition, collagen synthesis in skin fibroblasts plays a major role in skin rejuvenation[14], while the synthesis of hyaluronic acid, HA, an unsulfated glycosaminoglycan, regulates skin hydration, wrinkles and tissue repair[15]. Our results indicate that the CMEO significantly induced collagen and hyaluronic acid synthesis from the fibroblasts, suggesting that the CMEO could enhance skin moisture and can result in skin being less dry by increasing HA synthesis[16].
Our results indicate that CMEO, by reducing the secretion of IL-6, IL-1β, MCP-1, and MIP-1α, decreases the mRNA synthesis of MMPs that are produced by fibroblasts and stimulate the synthesis of total collagen. We suggest that CMEO, which present a high content of geraniol, could be part of cosmetic formulations, with antiaging indication, since, in addition to increasing collagen synthesis, it decreases the inflammatory response, which triggers the expression of MMPs, contributing to the degradation of matrix collagen extracellular, fundamental findings for skin aging. Finally, the results showed the potential of CMEO, probably due to the action of geraniol, as an important cosmetic ingredient, although further studies are needed to better understand the action and regulation of signaling pathways in different biological processes.
MMP´s are enzymes that participate in the breakdown of elements in the extracellular matrix, playing an important role in homeostasis, aging and skin healing[17]. In addition to chronological aging, induced by UV radiation, increased production of MMPs, including MMP-1, MMP-2 and MMP-9, promotes changes in collagen synthesis by collagen induction or by degradation of the ECM[18]. The results obtained shows that the CMEO significantly reduced (p<0.05) the mRNA expression of the evaluated MMP´s, suggesting that CMEO could attenuate collagenolysis and elastolysis by matrix MMP´s that occur under normal conditions, and in most inflammatory processes[19]. The inflammatory response occurs, at least in part, by fragments of the proteolysis (e.g., elastin), which function as chemotactic agents, stimulating inflammation, proliferation, and angiogenesis[20].
The ability to reduce IL-6 secretion suggests that CMEO modulates collagenolytic effects through the negative modulation of this interleukin, where after induction by ultraviolet and infrared radiation, it stimulates, for example, the expression of MMP-1[21]. The reduction in the secretion of IL-1β, observed in treated fibroblasts with CMEO, can regulate the expression and activation of MMP´s[22], as MMP-2[23], and MMP-9 [24]. Regarding the evaluated chemokines, the results obtained after the treatment of fibroblasts with CMEO, suggest that the reduction in the levels of MCP-1 and MIP-1α may reduce the expression of MMP´s, since results described in the literature, indicate an increase in MMP-1 and MMP-9 by these chemokines[25,26]. The degradation of collagen and glycosaminoglycans, promotes considerable deterioration of connective tissue leading to the appearance of wrinkles, reduced skin elasticity and firmness[27].
Geraniol is a natural acyclic monoterpene alcohol found in the essential oils of lemon grass, palmarosa, citronella, and other plants[28]. It is used in cosmetics, shampoos, and other non-cosmetic products[29]. Recently, geraniol was shown to possess various pharmacological properties, including antioxidant, antimicrobial, antitumor, and anti-inflammatory activities[30]. The effects presented, could be attributed, at least in part, to the action of geraniol, since its anti-inflammatory effect[31,32], indicates that this component of CMEO it could negatively modulate the secretion of interleukins and chemokines, promoting less expression of enzymes related to collagenolysis and elastolysis, in addition to promoting an increase in total collagen synthesis. Thus, CMEO would act as a skin protector against the damages caused by chronological aging and/or induction by radiation exposure UV, since CMEO was not cytotoxic to fibroblast cell line, as well as in similar results described in the literature[33-35].
Conclusions
Our results showed that CMEO stimulated the synthesis of collagen and hyaluronic acid, reduced the secretion of inflammatory cytokines and negatively modulated MMP expression. These finds, associated with new studies, suggest a potential anti-aging, anti-scar, and would healing, especially in its inflammatory phase, from CMEO, through inflammatory control and possible prevention of degradation of the extracellular matrix. However, future studies with in vivo models should be conducted to validate the effects observed with CMEO.
Financing source
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Acknowledgements
The authors would like to thank WNF Essential Oils for kindly donating the CMEO evaluated in this research.
Contributors
Study design: CRO; RPV.
Data curation: CRO; RPV.
Data collect: CRO; RPV; MCD.
Data analysis: LMB; CRO; RPV.
Original manuscript writing: CRO; RPV; MCD; LMB.
Review writing and editing: CRO; RPV; LMB
Referências
1. Promila. A review on the medicinal and aromatic Plant Cymbopogon martinii (Roxb.) Watson (Palmarosa).Int J Chem Stud. 2018; 6(2): 1311-1315. ISSN: 2349–8528. [https://www.chemijournal.com/search/?q=A+review+on+the+medicinal].
2. Scherer R, Wagner R, Duarte MCT, Godoy HT. Composição e atividades antioxidante e antimicrobiana dos óleos essenciais de cravo-da-índia, citronela e palmarosa. Rev Bras Pl Med. 2009; 11: 442-49. ISSN 1516-0572. [https://doi.org/10.1590/S1516-05722009000400013].
3. Elshafie HS, Camele I. An Overview of the Biological Effects of Some Mediterranean Essential Oils on Human Health. Biomed Res Int. 2017. [https://doi.org/10.1155/2017/9268468].
4. Andrade BFMT, Conti BJ, Santiago KB, Fernandes Júnior A, Sforcin JM. Cymbopogon martinii essential oil and geraniol at noncytotoxic concentrations exerted immunomodulatory/anti-inflammatory effects in human monocytes. J Pharm Pharmacol. 2014; 66: 1491-6. [https://doi.org/10.1111/jphp.12278] [https://pubmed.ncbi.nlm.nih.gov/24934659/].
5. Wang J, Su B, Zhu H, Chen C, Zhao G. Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-κB and p38 MAPK. Exp Ther Med. 2016; 12: 3607–13. [https://doi.org/10.3892/etm.2016.3850] [https://pubmed.ncbi.nlm.nih.gov/28105094/].
6. Abdulkhaleq LA, Assi MA, Abdullah R, Zamri-Saad M, Taufiq-Yap YH, Hezmee MNM. The crucial roles of inflammatory mediators in inflammation: A review. Vet World. 2018; 11: 627–35. EISSN: 2231-0916. [https://doi.org/10.14202/vetworld.2018.627-635] [https://pubmed.ncbi.nlm.nih.gov/29915501/].
7. des Jardins-Park HE, Foster DS, Longaker MT. Fibroblasts and wound healing: an update. Regen Med. 2018; 13: 491-95. ISSN 1746-0751. [https://doi.org/10.2217/rme-2018-0073] [https://pubmed.ncbi.nlm.nih.gov/30062921/].
8. Gao H.M., Hong J.S. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008; 29: 357–65. [https://doi.org/10.1016/j.it.2008.05.002] [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4794280/].
9. Raina VK, Srivastava SK, Aggarwal KK, Syamasundar KV, Khanuja SPS. Essential oil composition of Cymbopogon martinii from different places in India. Flavour Fragr J. 2003; 18: 312-15. [https://doi.org/10.1002/ffj.1222].
10. Prashar A, Hili P, Veness RG, Evans, CS. Antimicrobial action of palmarosa oil (Cymbopogon martinii) on Saccharomyces cerevisiae. Phytochemistry. 2003; 63: 569–575. [https://doi.org/10.1016/S0031-9422(03)00226-7] [https://pubmed.ncbi.nlm.nih.gov/12809717/].
11. Randriamiharisoa RP, Gaydou EM. Composition of palmarosa (Cymbopogon martinii) essential oil from Madagascar. J Agr Food Chem. 1987; 35: 62-6. ISSN: 0021-8561 [https://doi.org/10.1021/jf00073a015].
12. Oliveira PF, Alves JM, Damasceno JL, Oliveira RAM, Dias Júnior H, Crotti AEM et al. Cytotoxicity screening of essential oils in cancer cell lines. Rev Bras Farmacogn. 2015; 25: 183-8. ISSN: 1981-528X. [https://doi.org/10.1016/j.bjp.2015.02.009].
13. Elsayed EA, Sharaf-Eldin MA, Wadaan M. In vitro Evaluation of Cytotoxic Activities of Essential Oil from Moringa oleifera Seeds on HeLa, HepG2, MCF-7, CACO-2 and L929 Cell Lines. Asian Pac J Cancer Prev. 2015; 16: 4671-75. [https://doi.org/10.7314/APJCP.2015.16.11.4671].
14. Binic I, Lazarevic V, Ljubenovic M, Mojsa J, Sokolovic D. Skin Ageing: Natural Weapons and Strategies. Evid Based Compl Alter Medv. 2013; 1: 27248. [https://doi.org/10.1155/2013/827248].
15. Rittie L, Fisher GJ. UV-light-induced signal cascades and skin aging. Ageing Res Rev. 2002; 1: 705-20. [https://doi.org/10.1016/S1568-1637(02)00024-7].
16. Papakonstantinou E, Roth M, Karakiulakis G. Hyaluronic acid: a key molecule in skin aging. Dermatoendocrinol. 2012; 4(3): 253-8. [https://doi.org/10.4161/derm.21923].
17. Tammi R, Pasonen-Seppanen S, Kolehmainen E, Tammi M. Hyaluronan synthase induction and hyaluronan accumulation in mouse epidermis following skin injury. J Invest Dermatol. 2005; 124(5): 898-905. [https://doi.org/10.1111/j.0022-202X.2005.23697.x].
18. West MD, Pereira-Smith OM, Smith JR. Replicative senescence of human skin fibroblasts correlates with a loss of regulation and overexpression of collagenase activity. Exp Cell Res. 1989: 184(1): 138-47. [https://doi.org/10.1016/0014-4827(89)90372-8].
19. Limtrakul P, Yodkeeree S, Thippraphan P. Anti-aging and tyrosinase inhibition effects of Cassia fistula flower butanolic extract. BMC Complement Altern Med. 2016; 16: 497. [https://doi.org/10.1186/s12906-016-1484-3].
20. Van Doren SR. Matrix metalloproteinase interactions with collagen and elastin. Matrix biology. Matrix Biol. 2015; 44-46: 224-31. [ https://doi.org/10.1016/j.matbio.2015.01.005 ].
21. Antonicelli F, Bellon G, Debelle L, Hornebeck W. Elastin-elastases and inflamm-aging. Curr Top Dev Biol. 2007; 79:99-155. [https://doi.org/10.1016/S0070-2153(06)79005-6].
22. Sundararaj KP, Samuvel DJ, Li Y, Sanders JJ, Lopes-Virella MF, Huang Y. Interleukin-6 released from fibroblasts is essential for up-regulation of matrix metalloproteinase-1 expression by U937 macrophages in coculture: cross-talking between fibroblasts and U937 macrophages exposed to high glucose. J biol chem. 2009; 284: 13714-24. [https://doi.org/10.1074/jbc.M806573200].
23. Schönbeck U, Mach F, Libby P. Generation of Biologically Active IL-1β by Matrix Metalloproteinases: A Novel Caspase-1-Independent Pathway of IL-1β. Processing J Immunol. 1998; 161(7): 3340-46. [https://doi.org/10.4049/jimmunol.161.7.3340].
24. Choi YA, Lee DJ, Lim HK, Jeong JH, Sonn JK, Kang SS, Baek SH. Interleukin-1β stimulates matrix metalloproteinase-2 expression via a prostaglandin E2-dependent mechanism in human chondrocytes. Exp Mol Med. 2004; 36(3): 226-32. [https://doi.org/10.1038/emm.2004.31].
25. Cheng CY, Kuo CT, Lin CC, Hsieh HL, Yang CM. IL-1beta induces expression of matrix metalloproteinase-9 and cell migration via a c-Src-dependent, growth factor receptor transactivation in A549 cells. Br J Pharmacol. 2010; 160(7): 1595–1610. [https://doi.org/10.1111/j.1476-5381.2010.00858.x] [https://pubmed.ncbi.nlm.nih.gov/20649564/].
26. Suzuki M, Hashizume M, Yoshida H, Shiina M, Mihara M. IL-6 and IL-1 synergistically enhanced the production of MMPs from synovial cells by up-regulating IL-6 production and IL-1 receptor I expression. Cytokine. 2010; 51(2): 178–83. [https://doi.org/10.1016/j.cyto.2010.03.017] [https://pubmed.ncbi.nlm.nih.gov/20403707/].
27. Robinson S, Scott K, Balkwill F. Chemokine stimulation of monocyte matrix metalloproteinase‐9 requires endogenous TNF‐α. Eur J Immunol. 2002; 32(2): 404-12. [https://doi.org/10.1002/1521-4141(200202)32:2<404::AID-IMMU404>3.0.CO;2-X] [https://pubmed.ncbi.nlm.nih.gov/11813159/].
28. Knott A, Reuschlein K, Mielke HV. Natural Arctium lappa fruit extract improves the clinical signs of aging skin. J Cosmet Dermatol. 2008; 7(4): 281–89. [https://doi.org/10.1111/j.1473-2165.2008.00407.x].
29. Bhattamisra SK, Hooi LP, Shyan LP, Chieh LB, Candasamy M, Sahu PS. Effect of geraniol and clarithromycin combination against gastric ulcers induced by acetic acid and Helicobacter pylori in rats. Phcog Res. 2019; 11(4): 356-62. [https://doi.org/10.4103/pr.pr_21_19].
30. Wang J, Su B, Zhu H, Chen C, Zhao G. Protective effect of geraniol inhibits inflammatory response, oxidative stress and apoptosis in traumatic injury of the spinal cord through modulation of NF-κB and p38 MAPK. Exp Ther Med. 2016; 12(6): 3607–13. [https://doi.org/10.3892/etm.2016.3850] [https://pubmed.ncbi.nlm.nih.gov/28105094/].
31. Lei Y, Fu P, Jun X, Cheng P. Pharmacological Properties of Geraniol: A Review. Pl Med. 2019; 85(1): 48-55. [https://doi.org/10.1055/a-0750-6907] [https://pubmed.ncbi.nlm.nih.gov/30308694/].
32. Huang Y, Yang XL, Ni YH, Xu ZM. Geraniol suppresses proinflammatory mediators in phorbol 12-myristate 13-acetate with A23187-induced HMC-1 cells. Drug Des Devel Ther. 2018; 12: 2897-2903. [https://doi.org/10.2147/DDDT.S145702] [https://pubmed.ncbi.nlm.nih.gov/30254419/].
33. Chen W, Viljoen AM. Geraniol — A review of a commercially important fragrance material. S Afr J Bot. 2010; 76(4): 643-51. [https://doi.org/10.1016/j.sajb.2010.05.008].
34. Sinha S, Jothiramajayam M, Ghosh M, Mukherjee A. Evaluation of toxicity of essential oils palmarosa, citronella, lemongrass and vetiver in human lymphocytes. Food Chem Toxicol. 2014; 68:71-7. [https://doi.org/10.1016/j.fct.2014.02.036] [https://pubmed.ncbi.nlm.nih.gov/24650756/].
35. Raina V, Srivastava SK, Aggarwal KK, Syamasundar KV, Khanuja SPS. Essential oil composition of Cymbopogon martinii from different places in India. Flavour Fragr J. 2008: 18: 312–15. [https://doi.org/10.1002/ffj.1222].