Artigo de Pesquisa
Isolation of active antiphytopathogen compound from extracts of Anadenanthera colubrina var. cebil (Griseb.) Altschul
Abstract
Several microorganisms are responsible for great economic losses in world agriculture. Preventive and treatment methods are applied to avoid contamination of crops by these microorganisms, however, the use of chemical antimicrobials damages health and the environment. Secondary plant metabolites are safe natural sources of antimicrobials for this application. Fabaceae family has its history described in the literature as a potential source for obtaining antimicrobial bioactive. The objective of this work was to isolate bioactive compounds guided by antimicrobial assays against bacteria and fungi in vitro. Organic extracts were prepared by eluotropic series of leaves of Anadenanthera colubrina var. cebil and were tested against six bacteria and six fungi phytopathogenic. The antimicrobial assays of minimum inhibitory concentration (MIC) and minimum microbicidal concentration (MMC) were performed at each purification step that occurred through HPLC-DAD, Flash Chromatography and HPLC-preparative analysis, to confirm the isolation of the bioactive. Through bioguided isolation, the compound p-hydroxybenzoic acid was obtained, which showed activity against the phytobacteria Xanthomonas campestris pv. campestris and Acidovorax citrulli.
- Keywords:
- Angico.
- Xanthomonas campestris.
- Acidovorax citrulli.
- HPLC-DAD.
- Flash Chromatography.
- HPLC-preparative.
Introduction
Vegetable diseases, caused by several pathogenic microorganisms, are one of the main problems faced by world agriculture[1]. Bacteria and fungi are among the main pathogens that cause a great decrease in productivity and consequently economic losses in this sector[2]. Among the methods applied to control diseases in the field are preventive methods, which have low efficiency, and treatment methods, such as the application of synthetic antimicrobials[3]. These have caused problems related mainly to damage to human and animal health, in addition to the accumulation of environmental contamination[4].
Secondary plant metabolites are natural sources of substances with antimicrobial properties, which can be used as an alternative in substitution to synthetics[5]. This strategy minimizes the negative impacts associated with being considered safe for health and the environment, in addition to being economically advantageous due to the low cost, they are also capable of reducing the impact of microorganisms on agriculture. In addition, plant extracts reduce the possibility of causing microbial resistance, as they are complex mixtures of metabolites[6].
Several studies have demonstrated how the species of the Fabaceae family have potential as sources of antimicrobials for a great diversity of pathogens[7-9]. Anadenanthera colubrina var. cebil (Griseb.) Altschul, popularly known as Angico, belonging to this family, it is widely used in folk medicine because it is related to the antimicrobial properties of its leaves[10,11]. This work aimed to conduct a bioguided study on organic extracts of A. colubrina var. cebil, to obtain compounds with antimicrobial activity against phytopathogenic bacteria and fungi.
Materials and methods
Plant material
Leaves of A. colubrina were collected in the Catimbau National Park (08°34′30,96″ S e 37°14′51,76″ W), in the Northeast of Brazil. The collected material was oven dried at 45ºC for 72 h, then ground to obtain a thin powder, stored in an airtight container and kept at 4ºC until use. One specimen identified and registered by the Herbarium Dárdano de Andrade-Lima of the Instituto Agronômico de Pernambuco (IPA), under voucher IPA - 80350. The plant material was registered in the Sistema Nacional de Gestão do Patrimônio Genético e do Conhecimento Tradicional Associado (SisGen) at number A08E18B.
Organic Extracts
A hundred grams of the powder of the leaves de A. colubrina subjected to eluotropic series of organic solvents: cyclohexane (CHX), chloroform (CHL), ethyl acetate (EtOAc) and methanol (MeOH) in Soxhlet, respecting the boiling temperature of each solvent, and each was kept under reflux for 24 h. The extracts obtained were then filtered (Whatman n°1), and the solvents were entirely removed on a rotary evaporator at 45°C under reduced pressure. The dry extracts were stored at 4°C hermetically sealed until use.
Phytopathogenic microorganisms
The organic extracts tested against 12 phytopathogens. Six bacteria: Acidovorax citrulli (Acc – strain Ac1.12), Pectobacterium carotovorum subsp. carotovorum (Pcc – strain Pcc31), Ralstonia solanacearum (Rsol – strain CM10R22), Xanthomonas campestris pv. campestris (Xcc – strain Xcc53), Xanthomonas campestris pv. malvacearum (Xcm – strain Xcv137) and Xanthomonas campestris pv. viticola (Xcv – strain Xcm11.2.1) obtained by the Collection of Cultures of the Phytobacteriology Laboratory of the Department of Agronomy of Universidade Federal Rural de Pernambuco (UFRPE), Brazil. For antimicrobial tests the isolates were grown in a nutrient medium of yeast dextrose - NYDA (5 g.L-1 Yeast extract; 3 g.L-1 meat extract; 5 g.L-1 peptone; 10 g.L-1 dextrose; 18 g.L-1 de agar) for 24 h at 30°C. Six fungi: Aspergillus flavus (Af – strain 6029), Fusarium moniliforme (Fm – strain URM - 5411), Fusarium oxysporum (Fo – strain URM - 6185), F. solani (Fs – strain URM – 6264), Rhizopus sotolonifer (Rs – strain URM – 6525) and Verticillium lecanii (Vl – strain URM – 6171), obtained from the mycological collection of Micoteca-URM of the Department of Mycology of Universidade Federal de Pernambuco (UFPE), Brazil. For antimicrobial tests, the isolates were grown in Potato Dextrose Agar - PDA (4 g.L-1 potato extract; 15 g.L-1 dextrose; 18 g.L-1 agar) for 5 days at 48°C.
Screening Antimicrobial Activity
To evaluate the antimicrobial activity of A. colubrina leaves, the organic extracts, fractions, and isolated compound were solubilized in an aqueous solution at a concentration of 100mg.ml-1 with 10% dimethyl sulfoxide (DMSO) and were sterilized by filtration through a microfilter 0.22 µm (GV-Millipore).
The minimum inhibitory concentration (MIC) was determined by the microdilution method (CLSI, 2011) with modifications. A serial dilution of the extract/fractions was prepared in NYD or BD and 15 μl (Absorbance 600 nm = 0.150 ± 0.05) of bacteria or fungi suspension was added. The concentration of the samples ranged from 50 mg.ml-1 to 100 µg.ml-1 for organic extracts, from 6 mg.ml-1 to 22 µg.ml-1 for fractions and 500 to 1 ug.ml-1 for purified compound. The samples were incubated for 24 h for bacteria and 48 h for fungi, at 30°C for both. As a positive control, chloramphenicol was used for bacteria, and cercobin for tested fungi and sterile water with DMSO (10%) was used as a negative control. All tests were performed in triplicate.
To determine the minimum bactericidal or fungicidal concentration (CMB or CMF) after the microplate incubation period, 5 µL of the solution from each well was transferred to NYDA plates and incubated again for the same period. The complete absence of growth on the agar surface with the lowest concentration of the sample was defined as the MBC or CMF, respectively for bacteria and fungi.
Flash Chromatography
The active organic extract was fractionated by flash chromatography (Biotage™ Isolera one, Biotage™, Charlotte, NC, EUA). The separation occurred in a 50 g SNAP KP-SIL column (company Biotage™, Charlotte, NC, EUA). The mobile phase was a gradient of N-hexane: EtOAc, 30:70 (v / v) with 1 column volume (1 CV); N-hexane: EtOAc, 30:70 to 0: 100, (v / v) with 6 CV; MeOH: EtOAc, 0: 100 to 20:80 (v / v) with 6 CV; MeOH: EtOAc, 20:80 to 80:20 (v / v) with 3 CV. The flow of the mobile phase had a flow rate of 70 ml.min-1 and scan detection from 200 to 800 nm. The 38 fractions were grouped by software that evaluates the fractions according to the UV absorption spectrum, resulting in 6 fractions.
High-performance liquid chromatography
The active extract and fraction, and the isolated compound were analyzed on HPLC-DAD (1260 infinity LC System-DAD, Agilent OpenLAB CDS EZChrom Edition software, version 04.05 of Agilent Technologies, Santa Clara, CA, USA) equipped with the Zorbax, SB-C18, 5 µm and 4.6 x 250 mm column and Zorbax SB-C18 pre-column of 5 µm and 4.6 x 12.5 mm. For this, samples at a concentration of 5 mg.ml-1 were solubilized in methanol and filtered through 0.22 µm polytetrafluoroethylene (PTFE) filters. The mobile phase was composed of the following solutions: (A) 0.3% acetic acid with Milli-Q water (Millipore) and (B) 100% acetonitrile (Merck).
The active extract was analyzed under exploratory chromatographic conditions using the linear-gradient method of 95 - 40% (A) between 0-30min, with a flow rate of 2.4 ml.min-1, the initial pressure of 202 Bar and ultraviolet detection (UV) from 196 to 400nm. The chromatographic conditions for analysis of the active fraction and the purified compound followed a linear gradient of 92 - 65% (A) 0-15 min, with a flow rate of 2.4 ml.min-1, the initial pressure of 202 Bar and detection at 256 nm. In both analyzes, the identified peaks had retention time and UV spectrum compared to the commercial reference standards: gallic acid, p-coumaric acid, caffeic acid, catechin, trans-ferulic acid, quercetin 3β D-glucoside, chlorogenic acid, quercetin, rutin, and ellagic acid.
Preparative High-performance liquid chromatography
To isolate the active compound, the active fraction obtained by the flash chromatography system had its compounds separated by AutoPurification HPLC System ™ (model: 2767 Sample Manager, 2545 Binary Gradient Module, System Fluidics Organizer, System Fluidics Organizer, 2489 UV / Vis Detector, MassLynx Software with FractionLynx Application Manager and ACQUITY QDa Detector - Waters). The fraction solubilized in methanol at a concentration of 15 mg.ml-1 and filtered through 0.22 µm PTFE filters. The separation and isolation of the compounds occurred through the preparative column XBridge Prep C18 (5 µm and 10 × 100 mm), through the following mobile phase; 0.1% formic acid in Milli-Q water (A) and 100% acetonitrile (B) (Merck) with a linear gradient from 94 to 65% (A) from 0 to 9 min; 9-10 min (65%-0% A), 10-14 min (0% A), 16-20 min (0% -94% A), at room temperature, flow rate 9 ml.min-1 and detection at 256 nm.
Results and Discussion
TABLE 1 shows the results obtained about the antimicrobial power of organic extracts from A. colubrina leaves against six phytopathogenic bacteria, according to the extraction solvent used. It is observed that all extracts showed reduced growth in the tested phytobacteria when compared to the control. However, it is observed that MIC ≤ 1.56 mg.ml-1 is registered in 66.6% of the phytobacteria in the ethyl acetate extract, followed by 33.3% in the chloroform and methanol extracts, and the cyclohexane extract showed no activity for any of the species. When evaluating the MBC, it is observed that only the ethyl acetate extract has bactericidal activity in concentrations ≤ 1.56 mg.ml-1 for A. citrulli and X. campestris pv. campestris.
| Extracts | Acc | Pcc | Rsol | Xcc | Xcm | Xcv | |
| CHX | MIC | 6.25 | 12.5 | 6.25 | 3.12 | 3.12 | 3.12 |
| MBC | 6.25 | 25 | 12.5 | 6.25 | 6.25 | 6.25 | |
| CHL | MIC | 3.12 | 6.25 | 1.56 | 1.56 | 3.12 | 3.12 |
| MBC | 6.25 | 12.5 | 3.12 | 1.56 | 3.12 | 6.25 | |
| EtOAc | MIC | 1.56 | 3.12 | 1.56 | 0.78 | 3.12 | 1.56 |
| MBC | 1.56 | 3.12 | 3.12 | 1.56 | 3.12 | 3.12 | |
| MeOH | MIC | 1.56 | 6.25 | 3.12 | 1.56 | 3.12 | 12.5 |
| MBC | 3.12 | 6.25 | 3.12 | 3.12 | 6.25 | 12.5 | |
| Chloramphenicol | MIC | 0.009 | 0.039 | 0.078 | 0.019 | 0.039 | 0.004 |
| MBC | 0.009 | 0.078 | 0.156 | 0.019 | 0.039 | 0.004 | |
| Legend: Acidovorax citrulli (Acc), Pectobacterium carotovorum subsp. carotovorum (Pcc), Ralstonia solanacearum (Rsol), Xanthomonas campestris pv. campestris (Xcc), X. campestris pv. malvacearum (Xcm) e X. campestris pv. viticola (Xcv); Cyclohexane extract (CHX); Chloroform extract (CHL); Ethyl acetate extract (EtOAc); Methanolic extract (MeOH). | |||||||
The antifungal activity of the extracts against phytopathogenic fungi shown in TABLE 2. It observed that there was a reduction in growth in the tested concentrations when compared to the control, however, no extract was considered to have relevant inhibitory or antifungal activity due to all results being higher than 6.25 mg.ml-1. In both microbiological tests, it was observed that the DMSO used to solubilize the organic extracts in an aqueous medium, did not affect the bacterial or fungal growth in the negative controls in the concentration used.
| Extracts | Af | Fm | Fo | Fs | Rs | Vl | |
| CHX | MIC | 6.25 | 12.5 | 25 | 6.25 | 6.25 | 6.25 |
| MFC | 6.25 | 12.5 | 50 | 12.5 | 6.25 | 6.25 | |
| CHL | MIC | 12.5 | 12.5 | 50 | 6.25 | 12.5 | 6.25 |
| MFC | 12.5 | 25 | 50 | 12.5 | 12.5 | 12.5 | |
| EtOAc | MIC | 12.5 | 12.5 | 25 | 6.25 | 12.5 | 6.25 |
| MFC | 12.5 | 12.5 | 25 | 12.5 | 12.5 | 12.5 | |
| MeOH | MIC | 12.5 | 25 | 50 | 6.25 | 12.5 | 12.5 |
| MFC | 12.5 | 25 | 50 | 12.5 | 12.5 | 12.5 | |
| Cercobin | MIC | 0.12 | 0.12 | 0.5 | 0.25 | 0.12 | 0.12 |
| MFC | 0.12 | 0.25 | - | 0.25 | 0.25 | 0.25 | |
| Legend: Fm: Fusarium moniliforme; Fo: Fusarium oxysporum; Fs: Fusarium solani; Rs: Rhizopus sotolonifer; Vl: Verticillium lecanii; CHX: Cyclohexane extract; CHL: Chloroform extract; EtOAc: Ethyl acetate extract; MeOH: Methanolic extract. | |||||||
From the results obtained in the screening of antimicrobial activity, bioguided purification of the bioactive compound was continued. Given the observed, the EtOAc extract was selected to be analyzed on HPLC-DAD (FIGURE 1). This analysis allowed the detection of six main peaks (≥ 500 mAU), between the retention time (Rt) 3 and 4 min, peak 1 (λmax 228, 260 and 294), between Rt 4 and 5 min, peak 2 (λmax 256), between 7 and 8 min, peak 3 (λmax, 265, 354) peak 4 (λmax 256, 352) and peak 5 (λmax 256, 356), and between 10 and 11 min peak 6 (λmax 256 and 370). Peak 2 is the major compound of the extract. Peak 6, when compared to retention time (Rt) and UV absorption spectrum, was identified as quercetin, in addition to traces of catechin and gallic acid were also identified. Except for peak 6, it was not possible to identify the other peaks obtained in the HPLC-DAD analysis by the reference standards used.

The EtOAc extract was subjected to Flash Chromatography (Biotage™), this semi purification generated six fractions grouped according to the UV absorption spectrum (FIGURE 2). These were tested against the microorganisms Acc and Xcc according to the activity recorded for the crude EtOAc extract (TABLE 3). Fraction 3 for the Xcc bacteria showed activity, as it had a MIC less than 1 mg.ml-1, so it was selected for the purification of the active compound.

| Fractions | Acc | Xcc | |
| Flash Fraction 1 | MIC | >5.8 | >5.8 |
| MBC | >5.8 | >5.8 | |
| Flash Fraction 2 | MIC | >5.8 | >5.8 |
| MBC | >5.8 | >5.8 | |
| Flash Fraction 3 | MIC | 1.45 | 0.72 |
| MBC | 1.45 | 1.45 | |
| Flash Fraction 4 | MIC | 5.8 | 5.8 |
| MBC | 5.8 | 5.8 | |
| Flash Fraction 5 | MIC | >5.8 | >5.8 |
| MBC | >5.8 | >5.8 | |
| Flash Fraction 6 | MIC | >5.8 | >5.8 |
| MBC | >5.8 | >5.8 | |
| Legend: Acidovorax citrulli (Acc), Xanthomonas campestris pv. campestris (Xcc). | |||
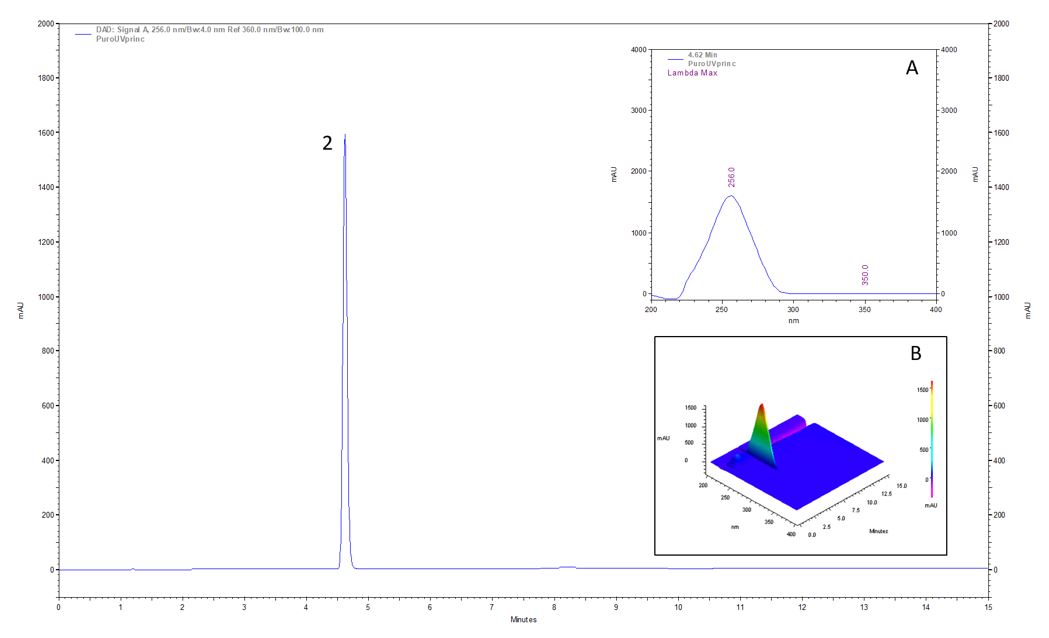
Thus, flash fraction 3 was analyzed and was subfractionated by HPLC-Preparative, giving rise to six subfractions that were again evaluated for their antimicrobial activity (TABLE 4). Subfraction 3 showed activity with MIC and MBC of 0.5 mg.ml-1 for Xcc. In view of its antibacterial potential, subfraction 3 was analyzed by HPLC-DAD, and a pure compound was detected. The UV spectrum associated with data available in the literature indicates that this compound is p-hydroxybenzoic acid (FIGURE 3), tracked through its UV absorption spectrum using flash chromatography (Biotage™) and HPLC-Preparative (Autopurification System™).
| Subfractions | Xcc | |
| Preparatory Subractions 1 | MIC | >0.5 |
| MBC | >0.5 | |
| Preparatory Subfractions 2 | MIC | >0.5 |
| MBC | >0.5 | |
| Preparatory Subractions 3 | MIC | 0.5 |
| MBC | 0.5 | |
| Preparatory Subfractions 4 | MIC | >0.5 |
| MBC | >0.5 | |
| Preparatory Subfractions 5 | MIC | >0.5 |
| MBC | >0.5 | |
| Preparatory Subfractions 6 | MIC | >0.5 |
| MBC | >0.5 | |
| Legend: Xanthomonas campestris pv. campestris (Xcc). | ||
Given the importance of developing a biopesticide, several studies have been carried out, in search of this objective, thus, a screening carried out by Silva et al.[11] with several plants of medicinal importance against several phytopathogens pointed to A. colubrina as an important source of antimicrobial compounds. From this result, a bioguided purification approach was carried out, starting with an extraction following the eluotropic order of solvents and using an in vitro anti-phytopathogenic bioassay. According to Santos et al.[12] to fight bacteria, an extract with MIC > 2.0 mg.ml-1 is considered inactive, therefore, the active and promising extract for the isolation of bioactive compound was the one with the lowest MIC and MBC values. According to this criterion, the EtOAc extract was the most active, with MIC and MBC ≤ 1.56 mg.ml-1 against the bacteria A. citrulli and X. campestris pv. campestris.
HPLC-DAD analysis of the crude EtOAc extract revealed a phenolic acid as the major (peak 2), with λ max 256 nm, it's ultraviolet (UV) absorption spectrum being very similar to p-hydroxybenzoic acid[13-17]. This acid was previously isolated and identified in Anadenanthera colubrina in the works of Gutierrez-Lugo et al.[18] and Weber et al.[19]. It was also possible to verify the presence of four flavonoids (FIGURE 1) with characteristic UV spectra. The UV spectrum of flavonoids shows two main peaks in the region of 240-400 nm, these two peaks are referred to as the band I of the molecule (usually 300-380nm) and band II (usually 240-280 nm). Thus the peaks 3 (λ max, 265, 354), 4 (λ max 256, 352) and 5 (λ max 256, 356) are referred to as quercetin derivatives and maybe quercetin 3,7-O-diglucoside (λ max 256, 355), quercetin 3-O-glucoside 7-O-rhamnoside (λ max 257,358), quercetin 3-methyl ether (λ max 257,358), quercetin 3-O-glucoside 7-O-rutinoside (λ max 257,358) among others[20]. Peak 6 λ max 256 and 370 identified through the standard, as already mentioned, is quercetin.
The purification of the bioguided EtOAc extract by the assay against phytopathogens in vitro resulted in the isolation (FIGURE 2) of the major component (peak 2 λ max 256) which when tested separately presented MIC and MBC of 0.5 mg.ml-1 X. campestris pv. campestris. The difference between the MIC and MBC values of the extract and the isolated substance may be due to the presence of nutritional components, common in extracts, such as proteins and sugars, which can contribute to the development of the microorganism[21]. Our data are in agreement with the findings of Araújo et al.[22] which isolated and identified by nuclear magnetic resonance (NMR) p-hydroxybenzoic acid the major compound of the ethyl acetate extract of aerial parts of A. colubrina.
Phenolic acids are part of the group of phenolic compounds, rarely occur as free acids, are divided into benzoic, cinnamic acids and their derivatives, p-hydroxybenzoic acid is the simplest form found in nature[23]. Despite data on the antimicrobial effects of phenolic acids[24,25], studies dealing with the anti-phytopathogenic properties of its metabolites or derivatives are still scarce. Literature data indicate that p-hydroxybenzoic acid inhibits the growth of plant pathogens. Cho et al.[26] found that the acid inhibits the growth of X. campestris with an IC50 of 0.136 mg.ml-1. However research indicates that the phytopathogen Xcc may have developed a functional degradation pathway of 4-HBA (4-hydroxybenzoate) that plays a role in detoxifying phenolic metabolites in the host during infection, however, the mechanistic details and the biological significance of this phenomenon have yet to be elucidated[27].
In fungi assays, all extracts were ≥ 6.25 mg.ml-1, however, the CHX extract was the most active with MIC and MBC of 6.25 mg.ml-1 for Aspergillus flavus, Rhizopus sotolonifer, and Verticillium lecanii, the rest of the extracts presented MIC and MFC ≥ 12.5 mg.ml-1. Campos et al.[28] perform a bioguided assay with A. colubrina against fungi and identify or extract hexane as the most active, assign an antimicrobial activity to three substances, among them: β-sitosterol and β-sitosterol linoleate, both in fruits and leaves and with MICs of 0.25 and 0.5 mg.ml-1 in front of Alternaria alternata respectively. Due to the similarity with ergosterol, steroidal substances can compete with fungal proteins involved in the synthesis of this metabolite and can be lethal to the fungus[28].
Conclusion
The bioguided approach carried out with the extracts of the leaves of A. colubrina through in vitro antimicrobial tests led to p-hydroxybenzoic acid which, in addition to being the major component of the active extract, showed antimicrobial activity against the phytopathogens Xanthomonas campestris pv. campestris with MIC and MBC of 0.5 mg.ml-1. The data highlight the potential of the species as an alternative source of this compound to fight diseases of economic importance in agriculture.
Acknowledgements
The authors are grateful to the financial support of the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE).
References
1. He DC, Zhan JS, Xie LH. Problems, challenges and future of plant disease management: From an ecological point of view. J Integr Agric. 2016; 15(4): 705-15. [http://dx.doi.org/10.1016/S2095-3119(15)61300-4].
2. Kotan R, Cakir A, Dadasoglu F, Aydin T, Cakmakci R, Ozer H et al. Antibacterial activities of essential oils and extracts of Turkish Achillea, Satureja and Thymus species against plant pathogenic bacteria. J Sci Food Agric. 2010; 90(1): 145-60. [http://dx.doi.org/10.1002/jsfa.3799] [https://pubmed.ncbi.nlm.nih.gov/20355025/].
3. Potnis N, Timilsina S, Strayer A, Shantharaj D, Barak JD, Paret ML et al. Bacterial spot of tomato and pepper: Diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol Plant Pathol. 2015; 16(9): 907-20. [http://dx.doi.org/10.1111/mpp.12244] [https://pubmed.ncbi.nlm.nih.gov/25649754/].
4. Kumar D, Chand R, Prasad LC, Joshi AK. A new technique for monoconidial culture of the most aggressive isolate in a given population of Bipolaris sorokiniana, cause of foliar spot blotch in wheat and barley. World J Microbiol Biotechnol. 2007; 23(11): 1647-51. [http://dx.doi.org/10.1007/s11274-007-9410-y].
5. Venturoso LR, Bacchi LMA, Gavassoni WL, Conus LA, Pontim BCA, Bergamin AC. Atividade antifúngica de extratos vegetais sobre o desenvolvimento de fitopatógenos. Summa Phytopathol. 2011; 37(1): 18-23. [http://dx.doi.org/10.1590/S0100-54052011000100003].
6. Tintino SR, Abel AA, Menezes IRA, Cícera CD, Coutinho HDM. Atividade antimicrobiana e efeito combinado sobre drogas antifúngicas e antibacterianas do fruto de Morinda citrifolia L. Acta Biol Colomb. 2015; 20(3): 193-200. [http://dx.doi.org/10.15446/abc.].
7. Barrera-Necha L, Bautista-Baños S, Bravo-Luna L, Bermúdez-Torres K, García-Suárez F, Jiménez-Estrada M et al. Antifungal activity against postharvest fungi by extracts and compounds of Pithecellobium dulce seeds (huamuchil). Acta Hortic. 2003; 628(12): 761-6. [http://dx.doi.org/10.17660/ActaHortic.2003.628.96].
8. Baños SB, Necha LLB, Lauzardo ANH, del Valle MGV, Tejacal IA, Sánchez DG. Powders, extracts and fractions of leaves of Cestrum nocturnum L. and their antifungal activity over two isolations of Fusarium spp. Rev Cient UDO Agric. 2008; 8(1): 42-51. [https://www.researchgate.net/publication/38107083].
9. Barrera-Necha LL, Baños SB, Luna LB, Suárez FJLG, Solano DA, Chilpa RR. Antifungal Activity of Seed Powders, Extracts, and Secondary Metabolites of Pachyrhizus erosus (L.) Urban (Fabaceae) Against Three Postharvest Fungi. Rev Mex Fitopatol. 2004; 22(3): 356-61. [https://www.redalyc.org/articulo.oa?id=61222307].
10. Palmeira JD, Ferreira SB, Souza JH, Almeida JM, Figueiredo MC, Pequeno AS et al. Evaluation of the antimicrobial activity in vitro and determination of minimum the inhibitory con- centration (MIC) of hidroalcoholicoc extracts of angico in strains Staphylococcus aureus. Rev Bras Análises Clín. 2010; 42(1): 33-7. [https://www.researchgate.net/publication/324017536].
11. Silva CMA, Costa BMSC, Silva AG, Souza EB, Silva MV, Correia MTS et al. Antimicrobial activity of several Brazilian medicinal plants against phytopathogenic bacteria. African J Microbiol Res. 2016; 10(17): 578-83. [http://dx.doi.org/10.5897/AJMR2014.6999].
12. Santos DKD do N, Almeida VS de, Araujo DRC de, Harand W, Soares AK de A, Moreira LR et al. Evaluation of cytotoxic, immunomodulatory and antibacterial activities of aqueous extract from leaves of Conocarpus erectus Linnaeus (Combretaceae). J Pharm Pharmacol. 2018; 70(8): 1092-101. [http://dx.doi.org/10.1111/jphp.12930] [https://pubmed.ncbi.nlm.nih.gov/29744882/].
13. Kowalski R, Wolski T. Evaluation of phenolic acid content in Silphium perfoliatum L. leaves, inflorescences and rhizomes. Electron J Polish Agric Univ. 2003; 6(1): 1-10. [https://www.researchgate.net/publication/324440956].
14. Mincea MM, Lupşa IR, Cinghiţå DF, Radovan C V, Talpos I, Ostafe V. Determination of methylparaben from cosmetic products by ultra performance liquid chromatography. J Serbian Chem Soc. 2009; 74(6): 669-76. [http://dx.doi.org/10.2298/JSC0906669M].
15. Okamoto Y, Hayashi T, Matsunami S, Ueda K, Kojima N. Combined activation of methyl paraben by light irradiation and esterase metabolism toward oxidative DNA damage. Chem Res Toxicol. 2008; 21(8): 1594-9. [http://dx.doi.org/10.1021/tx800066u] [https://pubmed.ncbi.nlm.nih.gov/18656963/].
16. Rasmussen H, Mogensen KH, Jeppesen MD, Sørensen HR, Meyer AS. 4-Hydroxybenzoic acid from hydrothermal pretreatment of oil palm empty fruit bunches – Its origin and influence on biomass conversion. Biomass and Bioenergy. 2016; 93(10): 209-16. [http://dx.doi.org/10.1016/j.biombioe.2016.07.024].
17. Sannino F, Sansone C, Galasso C, Kildgaard S, Tedesco P, Fani R et al. Pseudoalteromonas haloplanktis TAC125 produces 4-hydroxybenzoic acid that induces pyroptosis in human A459 lung adenocarcinoma cells. Sci Rep. 2018; 8(1):1-10. [http://dx.doi.org/10.1038/s41598-018-19536-2].
18. Gutierrez-Lugo MT, Deschamps JD, Holman TR, Suarez E, Timmermann BN. Lipoxygenase inhibition by anadanthoflavone, a new flavonoid from the aerial parts of Anadenanthera colubrina. Planta Med. 2004; 70(3): 263-5. [http://dx.doi.org/10.1055/s-2004-818920] [https://pubmed.ncbi.nlm.nih.gov/15114507/].
19. Weber CR, Soares CML, Lopes ABD, Silva TS, Nascimento MS, Eulália et al. Anadenanthera colubrina: um estudo do potencial terapêutico. Rev Bras Farm. 2011; 92(4): 235-44. [https://www.researchgate.net/publication/313612512_Anadenanthera_colubrina_A_therapeutic_potential_study].
20. Mabry TJ, Markham KR, Thomas MB, Mabry TJ, Markham KR, Thomas MB. The Ultraviolet Spectra of Flavones and Flavonols. Syst Identif Flavonoids. 1970; (see 111): 41-164. [http://dx.doi.org/10.1007/978-3-642-88458-0].
21. Andrade JC, Silva ARP, Freitas MA, Azevedo Ramos B, Freitas TS, Santos F de AG dos et al. Control of bacterial and fungal biofilms by natural products of Ziziphus joazeiro Mart. (Rhamnaceae). Comp Immunol Microbiol Infect Dis. 2019; 65(6): 226-33. [https://doi.org/10.1016/j.cimid.2019.06.006].
22. Araújo DRC, Silva TD, Harand W, Lima CSA, Neto JPF, Ramos BA et al. Bioguided purification of active compounds from leaves of Anadenanthera colubrina var. cebil (griseb.) Altschul. Biomolecules. 2019; 9(10): 1-14. [http://dx.doi.org/10.3390/biom9100590] [https://pubmed.ncbi.nlm.nih.gov/31597408/].
23. Arnoso BJM, Costa GF, Schmidt B. Biodisponibilidade e classificação de compostos fenólicos. Nutr Bras. 2019; 18(1): 39. [http://dx.doi.org/10.33233/nb.v18i1.1432].
24. Gañan M, Martínez-Rodríguez AJ, Carrascosa A V. Antimicrobial activity of phenolic compounds of wine against Campylobacter jejuni. Food Control [Internet]. 2009; 20(8): 739-42. [http://dx.doi.org/10.1016/j.foodcont.2008.09.012].
25. Merkl R, Hrádková I, Filip V, Šmidrkal J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J Food Sci. 2010; 28(4): 275-9. [http://dx.doi.org/10.17221/132/2010-CJFS].
26. Cho JY, Moon JH, Seong KY, Park KH. Antimicrobial activity of 4-hydroxybenzoic acid and trans 4-hydroxycinnamic acid isolated and identified from rice hull. Biosci Biotechnol Biochem. 1998; 62(11): 2273–6. [http://dx.doi.org/10.1271/bbb.62.2273] [https://pubmed.ncbi.nlm.nih.gov/9972252/].
27. Wang JY, Zhou L, Chen B, Sun S, Zhang W, Li M et al. A functional 4-hydroxybenzoate degradation pathway in the phytopathogen Xanthomonas campestris is required for full pathogenicity. Sci Rep. 2015; 5(8): 1-13. [http://dx.doi.org/10.1038/srep18456].
28. Campos VA, Perina FJ, Alves E, Sartorelli J, Moura AM, Oliveira DF. Anadenanthera colubrina (Vell.) Brenan produces steroidal substances that are active against Alternaria alternata (Fr.) Keissler and that may bind to oxysterol-binding proteins. Pest Manag Sci. 2014; 70(12): 1815-22. [http://dx.doi.org/10.1002/ps.3722] [https://pubmed.ncbi.nlm.nih.gov/24408227/].