Artigo de Pesquisa
Anatomical characterization of the vegetative organs of three Solanum sp. L. Juss. (Solanaceae) species popularly known as medicinal
Abstract
Neotropical forests have many plant species of great value, be it nutritional, ornamental and, mainly, medicinal. The substances present in these species are the most varied and many families are very relevant in this regard. The family Solanaceae comprises many species whose secondary metabolites, including alkaloids, make them an interesting pharmacological target. However, the large number of species in the family and their often, which are often morphologically similar, makes their therapeutic use unsafe. Thus, this study aimed to contribute to the anatomical characterization of the family to assist in the correct identification of species so as to support their safe therapeutic use. The analyzed species (S. paniculatum, S. sisymbriifolium and S. americanum) were collected in Atlantic forest environments and anatomical analyses were performed according to usual protocols in plant anatomy. The results confirmed important diagnostic characteristics for the distinction of the species and provided data that contribute to their safer and more effective use, since their anatomical characterization can be used as a reference to assist in the identification of species.
- Keywords:
- Jurubeba.
- Pharmacobotany.
- Solanaceae.
- Solanum americanum.
- Solanum paniculatum.
- Solanum sisymbriifolium.
Introduction
The occurrence and use of plants as well as the substances present in them are subjects that arouse great interest in the academic environment, in pharmaceutical industries, and among the population. However, despite their benefits, as some plants produce toxic substances, their use may be harmful for humans. Many plants are commonly used by the population without any prior knowledge and are generally commercialized as natural products without instructions about their therapeutic use, being the popular name of the species, the only information provided.
Even today, in several poor regions and even in large cities in the world, medicinal plants are sold in open markets, marketplaces, houses, and herbalist's shops, and grown in residential backyards. Reports and popular observations of medicinal plants contribute significantly to the dissemination of the therapeutic use of plants. Such use is usually empirically indicated, but many of the chemical constituents responsible for the therapeutic effects of these plants are still unknown[1].
In addition to the issue of production of toxic substances, the correct botanical identification of medicinal species is fundamental to develop pharmacological research, as well as to ensure the genetic conservation and maintenance of biodiversity[2] and correct and safe use by the population.
In Brazil, the registration of herbal medicines is regulated by the Resolution of the Collegiate Board - RDC nº 26 of May 13, 2014, which provides for the registration of herbal medicines and report of traditional herbal products. Thus, the identification and morpho-anatomical characterization of plants are fundamental for the quality control of the raw material used in the preparation of herbal medicines, as a guarantee of reliability [5]. It is also worth mentioning that researches on medicinal plants must meet standards of scientific rigor so that the production can be released[3], and such standards can only be obtained when professionals in the area are involved in the research.
Solanaceae family comprises 98 genera and around 2700 species, many of high economic value [4], and is widely used for therapeutic purposes due to its secondary metabolites[5]. Many species of this family present alkaloids. Thus, the family has species that serve as a source of medicines, but also species that can be toxic[6].
Within the family, the genus Solanum L. is one of the most researched and explored due to its wide number of species and diverse chemical composition, which includes a variety of steroidal saponins and glycoalkaloids of importance in the natural resistance of these plants against many pests[7].
However, due to the large number of species of Solanaceae, the terminological issue becomes very complex. Several species are known by the same popular names, which makes their therapeutic use risky. Because of that, they end up being contraindicated to treat diseases. There are also conflicts as to the plant organs and the stage of development at which they should be used.
In order to contribute to the anatomical study of the family Solanaceae and assist in the correct identification of species, so as to increase the reliability of their therapeutic use, this study aimed to make an anatomical characterization of the vegetative organs of three Solanum species used in folk medicine.
Material and methods
The species studied in this work were Solanum paniculatum L., Solanum sisymbriifolium Lam. and Solanum americanum Mill.. S. paniculatum, commonly known as jurubeba, is widely used as a medicinal plant to treat various diseases, such as respiratory problems, liver diseases, anemia, and as a tonic[8]. S. sisymbriifolium, also known as joá, is considered an infesting plant due to the easy dispersion of seeds and for having a chemical compound that makes it resistant to pests and diseases[9]. It is popularly used as a medicine for digestive and urinary problems[10]. S. americanum, also called aguaraguá, is used as medicine by the population in the treatment of injuries caused by Leishmania sp. (Trypanosomatidae)[7], cough, and infection by protozoa[2].
The study material was collected in an Atlantic forest environment at the A.C. Simões campus of the Federal University of Alagoas (UFAL) with the presence of reproductive structures. Three adult individuals of each species were collected and a representative part of the material was pressed for production of exsiccates, confirmation of identification, and creation of vouchers for deposit at the MAC herbarium with the following information: Campus - UFAL, Maceió AL, 02/02/2017, fl./fr., M.C.S. Esteves 2, 3, (MAC64076, MAC64077, MAC 57989).
Other parts of the material were fragmented and sorted into roots, stems and leaves and fixed in FAA 70 (formaldehyde, acetic acid, 70% ethanol, 1:1:18 v/v) for 24 hours[11]. After this time interval, the material was stored in 70% alcohol for further processing.
For anatomical analyses, free-hand cross sections were made of plant organs at various stages of development with the aid of a razor blade. They were clarified with 2% sodium hypochlorite, stained with 0.5% Astra blue aqueous solution and 0.5% safranin aqueous solution[12], and mounted between slides and coverslips with 40% glycerin to prepare semipermanent histological slides.
For the detection of chemical compounds, the following tests were carried out: ferric chloride for detection of phenolic compounds[13]; ruthenium red staining for mucilage and pectin[14]; Sudan IV and Sudan black B staining for lipids[15]; zinc iodine chloride for starch[16].
After preparation of histological slides, the samples were analyzed and photographed using an Olympus BX51 Microscope with an Olympus DP25 camera, Olympus DP2-BSW software.
Results and Discussion
S. americanum (FIGURE 1A) presented leaf blades with uniseriate epidermis, stomata on both sides, uniseriate, four-celled, tector trichomes on both surfaces, in small quantities, and even more scarce on the abaxial surface (FIGURE 1B-C); dorsiventral mesophyll with a layer of palisade parenchyma and three to four layers of spongy parenchyma (FIGURE 1B); midrib with a biconvex outline with a more prominent abaxial surface, uniseriate epidermis, parenchymal cortex and amphicrival vascular cylinder, with rare peltate glandular trichomes on the abaxial surface (FIGURE 1D-E).
The analysis of the primary structure of the stem revealed two lateral wings (FIGURE 1F), uniseriate epidermis with few uniseriate single-celled non-glandular trichomes, few glandular trichomes formed by a head cell, a tapered median cell, a basal cell, and few peltate glandular trichomes (FIGURE 1G); internal to the epidermis, the first layer of the cortex was composed of parenchymal cells and the rest corresponded to two to three layers of angular collenchyma and, internally to them, two more layers of parenchyma cells (FIGURE 1H-I). The vascular cylinder was composed of bicollateral vascular bundles surrounding a wide parenchymatous pith (FIGURE 1H-I). In secondary growth (FIGURE 2A), the phellogen was installed and the epidermis was replaced by the periderm with one to two layers of phellem cells (FIGURE 2B). The cortex started to be composed of two cell layers of lamellar collenchyma and, internally to them, two to three layers of parenchyma cells, which undergo anticlinal divisions during organ growth in thickness (FIGURE 2A-B). The vascular cylinder was composed of primary phloem, secondary phloem, secondary xylem and primary xylem and, internally to it, primary phloem.
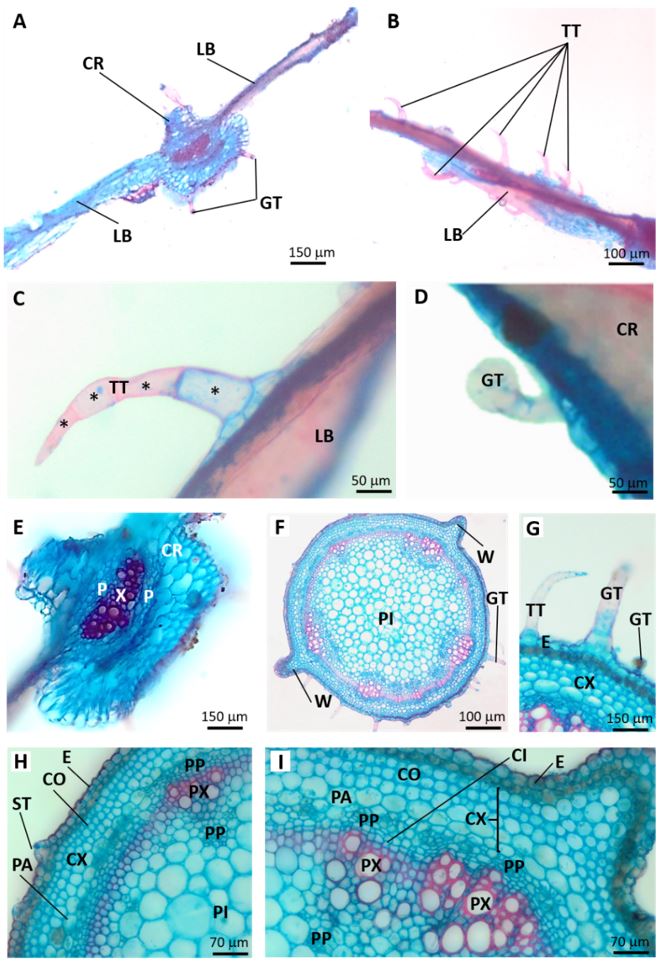
The root at initial secondary growth presented epidermis as outermost tissue, cortex with parenchyma cells of different sizes and shapes, phloem, and primary xylem, which was diarch (FIGURAS 2C-D). In secondary structure, the root presented the same cell types plus secondary phloem, cambium, and secondary xylem (FIGURE 2E-F).
In S. paniculatum, the leaves were hypostomatic and presented uniseriate epidermis, some capitate glandular trichomes on the adaxial surface (FIGURE 3A) and stellate non-glandular trichomes in great amount on the abaxial surface (FIGURE 3A-C) both on the midrib and the leaf blade; dorsiventral mesophyll composed of a layer of palisade parenchyma and three to four layers of spongy parenchyma; midrib with a biconvex outline with a more prominent abaxial surface, uniseriate epidermis, cortex with a layer of collenchyma and the rest of the layers of parenchyma, amphicrival vascular cylinder and horseshoe-shaped xylem (FIGURE 3C), and rare glandular trichomes on the adaxial surface (FIGURE 3D).

The primary structure of the stem showed a uniseriate epidermis with rare stellate non-glandular trichomes; cortex composed of approximately seven layers of parenchyma cells and, internally to them, some sclerified cells (FIGURE 4A). In initial secondary growth (FIGURE 4B), the stem started to present periderm and the cortex was composed of approximately seven layers of collenchyma cells, five layers of parenchyma cells, and sclerified cells. In well-established secondary growth (FIGURE 4C), the cortex presented the same cells as the previous stage but the layer of sclerified cells became continuous and there was the production of secondary xylem and phloem.
The primary structure of the root (FIGURE 4 D) presented epidermis, cortex formed by cell divisions in the endodermis and composed of six layers of rounded, uniform parenchymal cells, containing great amounts of starch. The vascular cylinder had primary phloem and xylem, the latter being tetrarch (FIGURE 4H). In secondary structure, at initial development, the installation of the phellogen and subsequent establishment of the periderm with five layers of phellem cells (FIGURE 4E) were observed; the cortex still contained starch and the vascular cylinder had additionally secondary phloem, cambium and secondary xylem (FIGURE 4 E-G).


S. sisymbriifolium presented amphistomatic leaves with few, uniseriate, uni-, bi- or tricellular non-glandular trichomes, both on the midrib and the leaf blade, on the two surfaces of the epidermis (FIGURE 5A-E); few stellate non-glandular trichomes only on the leaf blade, on both sides of the epidermis (FIGURE 5A-E); many glandular trichomes formed by a single head cell, a tapered median cell, and a basal cell distributed throughout both surfaces of the epidermis (FIGURE 5A-E); rare peltate glandular trichomes only on the lower surface of the midrib (FIGURE 5C); dorsiventral mesophyll with a layer of palisade parenchyma and three layers of spongy parenchyma (FIGURE 5E); midrib with a biconvex outline with a more prominent abaxial surface, uniseriate epidermis, cortex with three to four layers of collenchyma and the rest of parenchyma, and amphicrival vascular cylinder with horseshoe-shaped xylem (FIGURE 5A).
The primary structure of the stem presented uniseriate epidermis with few uniseriate, uni or bicellular non-glandular trichomes; cortex composed of three layers of parenchyma cells below the epidermis, six layers of angular collenchyma cells, and three to six layers of parenchyma cells (FIGURE 5F-G). The central cylinder presented bicollateral vascular bundles around the pit (FIGURE 5F-G), until the beginning of secondary growth (FIGURE 5H). In secondary growth, the epidermis was maintained; the cortex remained similar to that of the primary stem but with the appearance of a discontinuous band of sclerified cells surrounding the vascular cylinder, which was added with secondary phloem and xylem (FIGURE 5I-K).

In the initial of secondary structure of the root presented a uniseriate epidermis, a cortex composed of several layers of large, rounded parenchyma cells (FIGURE 6A), and a vascular cylinder with diarch primary xylem (FIGURE 6B). The inner cortex was formed by cells produced by meristematic activity of the layer of precursor cells of the endoderm, the proendodermis. This meristematic activity produces the internal cortex of the root, observed as conspicuous layers of stratified cells (FIGURE 6C-D). In the secondary structure, the phellogen was installed, and the periderm was developed (FIGURE 6C-D); the vascular cylinder started to present secondary phloem, cambium, and secondary xylem, with two protoxylem poles. The main anatomical differences are showed in the TABLE 1.
Regarding the histochemical tests performed, all reacted negatively in the three species.

| Charatcter | Foliar limb | Primary stem | Secondary stem | Root | |||||
|---|---|---|---|---|---|---|---|---|---|
| Trichomes | Central rib | ||||||||
| Species | Cortex | Vascular bundle | Wings | Trichomes | Cortex | Coating | Cortex | Protoxylem | |
| S. americanum | tt, gt -- -- |
par | bico | p | tt e gt - - |
col, par | periderm | col, par | 2 poles |
| S. paniculatum | stt, tg ++ - |
col, par | bico, ᴒ | a | stt -- | pa, scl | periderm | col, par, scl | 4 poles |
| S. sisymbrifolium | tt, stt, gt - - + |
col, par | bico, ᴒ | a | tt - |
col, par | epidermis | col, par, scl | 2 poles |
| Legend: Captions: a = absent; bico = biconvex; col = colenchyma; escl = slerenchyma; par = parenchyma; tt = tector tricichome; gt = glandulas Trichome glandular; stt = stellar tector trichome; p = present; --- = sparse; - = litle; + = many; ++ = too many; ᴒ = horseshoe shape. | |||||||||
The species studied here have different therapeutic properties. Thus, pointing out the anatomical differences between them is of paramount importance for their safe use.
Studies of the external and internal morphology of any plant used in medicine are extremely important for the identification of these species. Although some sophisticated chemical and molecular methods are available for identification of plant material, morphoanatomical studies are the simplest methods to prevent counterfeiting and adulteration of drugs[17].
In this work, it was found that the main anatomical differences were related to the type and quantity of trichomes present on the leaves and stems, mainly in the primary structure of these organs; the composition of the cortex of the midrib and the stem, in both primary and secondary structure; the shape of the vascular bundle in the midrib; the presence or absence of wings on the surface of primary stems; the outermost tissue of secondary stems; and the number of protoxylem poles of the root.
These differences do not only characterize the species, but also allow comparative analyses and represent diagnostic characteristics.
Trichomes are important structures in xeric environments, as they maintain a saturated atmosphere of water vapor around the leaf[18].
Trichomes can be glandular or non-glandular and were present in the three species studied. Glandular trichomes can be subdivided into capitate and peltate and present variable morphology, structure and density on the epidermis of different species and organs[19].
Both capitate and peltate glandular trichomes are frequently present in plants of the Asteraceae, Lamiaceae and Solanaceae families. Capitate trichomes usually consist of a basal cell, one to several stalk cells, and one or a few secretory cells at the tip of the stalk. They predominantly produce non-volatile or less volatile compounds that are directly exuded onto the surface of the trichome[20].
Peltate trichomes, of which typical examples can be found in mint and basil, consist of a basal cell, a (short) stalk cell, and a head composed of several secretory cells. Glandular trichomes are important in plant resistance against pests and can be used, therefore, as an important first line of defense against herbivorous insects and pathogens[21].
Other Solanum species also present glandular trichomes. For example, unbranched glandular trichomes were identified in S. lycopersicum L., in the twelve subsamples of the Vegetable Germplasm Bank (UFV)[22], and contained compounds such as glycosylated flavonoids (rutin), nitrogenous phenolic compounds (acid chlorogenic), methyl ketones (2-tridecanone and 2-undecanone), and zingiberene sesquiterpenes.
As for non-glandular trichomes, the literature provides information about their presence in other species of Solanum[23], and in some cases the dense layer of stellate trichomes on leaves has been interpreted as a bio-shield against stress[24].
In the present study, most trichomes of S. paniculatum were glandular, while those of S. sisymbriifolium were mostly non-glandular. In turn, both types were less frequent in S. americanum, which may suggest that this species is the most vulnerable of the three to herbivory and environmental stress.
Although ecological variations can affect the distribution of trichomes, the type of trichome is a stable trait in most species[21].
The morphology and distribution pattern of trichomes on leaf surfaces of the Solanum species analyzed by them were useful for distinction of the species, and that this would be especially useful for pharmacognosists, archaeobotanists, paleobotanists, and agronomists[22].
Regarding key anatomical features, the literature provides information on Solanum species that can be compared to the present findings, and when combined, they allow an accurate diagnosis and correct distinction and identification of the species. Solanum caavurana has been described as having leaves with a dorsiventral mesophyll, uniseriate palisade parenchyma, spongy parenchyma, and biconvex midrib. The initial secondary structure of the stem, in cross section, presents uni-stratified epidermis followed by collenchyma of the angular type, forming a continuous cylinder of four to five layers delimited by sclerenchyma bundles and five to six layers of cortical parenchyma. The vascular system is bicollateral with eustelic organization, delimited by a layer of discontinuous sclerenchyma bundles that surround the external phloem. The initial secondary structure of the root shows a poorly developed periderm and gradual suberization of epidermis cells. The cortical parenchyma consists of five to six layers of parenchyma cells[25].
Solanum lycocarpum presents stellate, bicellular, non-glandular and glandular trichomes on both sides of the leaf blade. The mesophyll has dorsiventral arrangement with palisade parenchyma formed by a layer of long, narrow cells, and spongy parenchyma formed by three to five layers of polyhedral cells that show a compact disposition, without typical characteristics of spongy parenchyma[26].
The leaves of S. crinitum present a uniseriate epidermis with multiseriate-stalked porrect-stellate trichomes. In cross section, the mesophyll is of the dorsiventral type with uniseriate palisade parenchyma and 4-5 layers of spongy parenchyma. The midrib shows a biconvex outline in cross section, with a more conspicuous convexity on the abaxial side. The epidermis is uniseriate with rounded cells and long-stalked stellate trichomes, similar to those of the leaf blade. Adjacent to the epidermis, there is the angular collenchyma, with about five layers, followed by the fundamental parenchyma.
In S. gomphodes, the leaves are amphistomatic and have uniseriate epidermis with sessile to subsessile, rarely stalked, porrect-stellate trichomes on the adaxial side, and long-stalked porrect-stellate trichomes on the abaxial side. The mesophyll, in cross section, is of the dorsiventral type, with uniseriate palisade parenchyma and 4-5 layers of spongy parenchyma. The midrib has uniseriate epidermis and stalked stellate trichomes.
The leaves of S. torvum have uniseriate epidermis with sessile stellate trichomes on both surfaces. The mesophyll is dorsiventral with uniseriate palisade parenchyma and 4-5 layers of spongy parenchyma. The midrib shows a single-celled layered epidermis followed by 4-7 layers of angular collenchyma. The stem has uniseriate epidermis with porrect-stellate trichomes. The collenchyma is angular forming a continuous cylinder, 5-8 layers, followed by a reduced cortical parenchyma, 4-5 layers. The vasculature consists of external phloem, xylem, and internal phloem. The root, in initial secondary growth, has an underdeveloped periderm and stratified parenchyma located between the periderm and the phloem. The secondary phloem and the xylem form a massive cylinder, although a tetrarch structure can be observed.
The mesophyll of S. pseudocapsicum reveals dorsiventral organization and is composed of one layer of palisade parenchyma and about four layers of parenchyma. In young stems, the epidermis consists of a single compact layer and most of the cortex is formed by parenchyma cells which accumulate starch grains[27].
In S. capsicoides, were observed amphistomatic leaves with uniseriate epidermis, with the presence, on both sides, of uniseriate, tetra-multicellular elongate, capitate and claviform glandular trichomes and uniseriate, bi-multicellular and hexa-multicellular elongate trichomes. The mesophyll has dorsiventral organization with one layer of cells of palisade parenchyma and spongy parenchyma. The midrib is biconvex, with a bicollateral vascular bundle surrounded by parenchyma, interrupted by angular collenchyma cells on both surfaces[28].
The above descriptions indicate that the main characteristics that vary between the Solanum species are the type, location and quantity of trichomes, as well as the number of protoxylem poles of the root.
Regarding trichomes, in S. sisymbriifolium, S. americanum and S. paniculatum, it was observed that the trichomes varied in type, quantity and location. These same variations were noticed in the analysis of the species described in the literature.
The number of protoxylem poles is a characteristic that had little divergence among the analyzed species: S. sisymbriifolium and S. americanum had two poles, and S. paniculatum had four. Among the other species found in the literature, this characteristic was described only in S. torvum, which had four poles.
A variation in the observedin the organization of the root stele of Pyrostegia venusta (Bignoniaceae) from triarch to heptarch[26]. In turn, in Pachyrhizus ahipa (Fabaceae), it was found that the organization of the root stele was consistently tetrarch[29].
Conclusion
The data obtained here from the anatomical analyses aimed at increasing the knowledge of Brazilian medicinal plants and of the family Solanaceae and allowing the better use of these plants, since their anatomical peculiarities can be used as a reference for the identification of the species.
The different patterns found in the type, location and quantity of trichomes, based on the present and literature data, indicate that this trait may be the most assertive for the distinction of species of the family Solanaceae.
The number of protoxylem poles also represented an important trait separating species; however, the intraspecific variations that may occur and the lack of comprehensive information in the literature on this aspect of the genus Solanum do not support the effective use.
Financing source
There was no financial support.
Conflict of interests
There is no conflict of interest between the authors.
Acknowledgements
The authors thank the Laboratory of Cellular and Molecular Biology of the Institute of Biological and Health Sciences (UFAL), coordinated by Professor Emiliano de Oliveira Barreto, for allowing us to use the equipment for microscopic analysis and photographic record.
Teacher Antônio Valeriano Pereira dos Santos, died in 2019, for his great contribution to Plant Anatomy in the State of Alagoas (BR). Despite his great talent and intellectual capacity, in his area of study, he preferred to dedicate himself entirely to the noble activity of teaching in the Bachelor and Degree Courses in Biological Sciences at the Institute of Biological and Health Sciences at the Federal University of Alagoas, contributing enormously with the training of students in Botany. In this way, the professor never obtained this number, which does not detract from his merit as a researcher and professor.
Contributors
Study design: AVPS; GC; JSV; MCSE
Data curated: AVPS; GC; JSV; MCSE
Data collect: GC
Data analysis: GC
Writing of the original manuscript: GC
Proofreading and Editing: GC.
Referências
1. Silva FP et al. Avaliação dos extratos de Anacardium occidentale Linn e Lippia sidoides Cham no processo de cicatrização tecidual. Estudo histológico em dorso de ratos. Braz J Period. 2013; 23: 18-25. [https://pesquisa.bvsalud.org/portal/resource/pt/biblio-853527?src=similardocs].
2. Cury G, Tomazello-Filho M. Caracterização e descrição da estrutura anatômica do lenho de seis espécies arbóreas com potencial medicinal. Rev Bras Pl Medic. 2011; 13: 311-318. [https://doi.org/10.1590/S1516-05722011000300010].
3. Ming LC. Estudos e pesquisas de plantas medicinais na agronomia. Hort Bras. 1994; 12: 3-9.
4. Brito SCD. Rodrigues W. Avaliação do marco regulatório na produção de medicamentos fitoterápicos no Brasil. Rev Pol Pub. 2016; 9: 531-538. [https://doi.org/10.18764/2178-2865.v19n2p531-538].
5. Olmstead RG, Bohs L. A summary of molecular systematic research in Solanaceae: 1982-2006. In: VI Genomics Meets Biodiversity. Belgium: International Society for Horticultural Science, 2007. 564 p. ISBN: 9789066054271.
6. Oliveira MLB, Cavalcante FS, Lima RA. Uso, classificação e diversidade de Solanum L. (Solanaceae). Rev Biodiv. 2020; 19(3): 142-159. [https://periodicoscientificos.ufmt.br/ojs/index.php/biodiversidade/article/view/10823].
7. Moreira HJC, Bragança HBN. Manual de Identificação de Plantas Infestantes (Hortifrutí). 1ªed. São Paulo: FMC Agricultural Products, 2011. 1017 p.
8. Friedman M, Rayburn JR, Bantle JA. Developmental toxicology of potato alkaloids in the frog embryo teratogenesis assay – Xenopus (FETAX). Food Chem Toxicol. 1991; 29: 537-547. [https://doi.org/10.1016/0278-6915(91)90046-A].
9. Cordeiro JMP, Félix LP. Conhecimento botânico medicinal sobre espécies vegetais nativas da caatinga e plantas espontâneas no agreste da Paraíba. Brasil. Rev Bras Pl Med. 2014; 16: 685-692. [https://doi.org/10.1590/1983-084x/13_077].
10. Victoria-Filho R. Controle de plantas daninhas em pastagens. In: Peixoto AM, Moura JC, Faria VP, eds. Pastagens na Amazônia. Piracicaba: FEALQ; 1986. 71-90.
11. Mentz LA, Lutzemberger LC, Schenkel EP. Da flora medicinal do Rio Grande do Sul: notas sobre a obra de D'Avila (1910). Cad Farm. 1997; 13: 25-47.
12. França F, Lago EL, Marsden PD. Plants Used in the Treatment of Leishmanial ulcers due to Leishmania (Viannia) braziliensis in an endemic area of Bahia, Brazil. Rev Soc Bras Medic Trop. 1996; 29: 229-232. [https://doi.org/10.1590/S0037-86821996000300002].
13. Johansen DA. Plant microtechnique. New York: Mc Graw - Hill Book; 1940.
14. Bukatsch F. Mikrokosmos. Comments on the Astra blue-safranin double staining. Mikrokosmos. 1972; 61: 61-225.
15. Pearse, AGE. Histochemistry: theoretical and applied. 3ªed. Baltimore: The Williams & Wilkins Company; 1972. 624 p.
16. Strasburger E. Kernteilungsbilder bei der Erbse. Flora Allg Bot Zeit. 1911; 102: 1-23. [https://doi.org/10.1016/S0367-1615(17)32334-0].
17. Fahn A. Structural and functional properties of trichomes of xeromorphic leaves. Ann Bot. 1986; 57: 631-637. [https://doi.org/10.1093/oxfordjournals.aob.a087146].
18. Ascensão L, Mota L, Castro MDM. Glandular trichomes on the leaves and flowers of Plectranthus ornatus: morphology, distribution and histochemistry. Ann Bot. 1999; 148: 221-227. [https://doi.org/10.1006/anbo.1999.0937].
19. Silva ML et al. Avaliação de tricomas em subamostras de tomate (Solanum lycopersicum L.). Enciclop Biosfera Centro Cient Conhecer. 2015; 11: 305. [https://conhecer.org.br/ojs/index.php/biosfera/article/view/1750].
20. Glas JJ et al. Plant glandular trichomes as targets for breeding or engneering of resistance to herbivores. Int J Mol Scie. 2012: 17077-17103. [https://doi.org/10.3390/ijms131217077].
21. Okpon ENU. Morphological notes on the genus Cassia. Edinb J Bot. 1969; 29: 185–196.
22. Adedeji O, Ajuwon OU, Babawale OO. Foliar epidermal studies, organographic distribution and taxonomic importance of trichomes in the family Solanaceae. Int J Bot. 2007; 3: 276-281, 2007. [https://doi.org/10.3923/ijb.2007.276.282].
23. Al Sheef NB. Micromorphology and ultrastructure of trichomes of libyan Salvia fruticosa Mill. Arch Biol Sci. 2015; 65: 239-248. [https://doi.org/10.2298/ABS1301239S].
24. Nurit-Silva K, Agra MF. Estudo Morfoanatômico de órgãos vegetativos de Solanum caavurana Vell. (Solanaceae). Latin American J Pharmacy. 2009; 28: 675-81. [http://sedici.unlp.edu.ar/handle/10915/7820].
25. Elias SRM et al. Anatomia foliar em plantas jovens de Solanum lycocarpum A. St.-Hil. (Solanaceae). Rev Bras Bot. 2003; 26: 169-174. [https://doi.org/10.1590/S0100-84042003000200004].
26. Sanghvi GV et al. Morpho-anatomy of Solanum pseudocapsicum. Braz J of Pharmacogn. 2011; 21: 11-15. [https://doi.org/10.1590/S0102-695X2011005000026].
27. Ferreira ARA et al. Leaf morphoanatomy of Solanum capsicoides All. (Solanaceae) from restinga area. Latin Am J Pharm. 2013; 32: 287-91. [https://www.researchgate.net/publication/289225163_Leaf_Morphoanatomy_of_Solanum_capsicoides_All_Solanaceae_from_Restinga_Area].
28. Gabrielli AC. Contribuição ao estudo anatômico da raiz de Pyrostegia venusta (Ker) Miers-Bignoniaceae. Rev Bras Bot. 1992; 15: 95-104.
29. Appezzato-da-Glória B, Estelita MEM. Development, structure and distribution of colleters in Mandevilla illustris and M. velutina (Apocynaceae). Rev Bras Bot. 2000; 23: 113-120. [https://doi.org/10.1590/S0100-84042000000200001].