Artigo de Pesquisa
Morphoanatomy and Histochemistry of three species of Paullinia L. (Sapindaceae)
Abstract
Paullinia is a monophyletic genus of lianas with a neotropical distribution, encompassing approximately 220 species. In Brazil, there are 102 species occurring mainly in the phytogeographic domains of the Atlantic Forest and Amazonia. The species of this genus have diverse uses as psychotropics, bactericides, antioxidants, fish poisons, stimulants and for ritualistic purposes. The genus is nevertheless insufficiently studied from the anatomical and histochemical standpoints. In the present study, morphoanatomical and histochemical investigations were made of the leaves of Paullinia dasygonia Radlk., P. micrantha Cambess. and P. trigonia Vell. The species studied have very similar leaf characters which makes their identification difficult. The leaves have dorsiventral structure, collateral vascular bundles and both non glandular and glandular trichomes which vary in the species as regards shape, length and distribution. The glandular trichomes have neutral polysaccharides and phenolic compounds. In addition, laticifers were observed containing lipid droplets, alkaloids, proteins and phenolic compounds in diverse regions of the leaf. Saponins, which are characteristic of the family, were observed in the secretory trichomes and generally in the mesophyll and midrib. The results obtained provide evidence justifying further pharmacological investigation of these three species, as well as others in the genus.
- Keywords:
- Paullinieae.
- Morphology.
- Leaves.
- Secretory structures.
Introduction
Paullinia L. is monophyletic, being one of the largest of the six genera of lianas within the tribe Paullinieae, which consists of approximately 220 species[1]. In Brazil, the genus is represented by 102 species occurring mainly in the Atlantic Forest and the Amazon domains[2], in humid environments with high insolation. The species of this genus have widespread ethnopharmacological uses and proven psychoactive, antibacterial and antioxidant effects[3]. A study that included 39 species of Paullinia, mentions that the plants are used for medicinal purposes in fresh or prepared form, as fish poisons, energy drinks, and for ritualistic purposes, amongst other uses[4]. However, there are few studies that address the anatomical description and histochemistry of the leaves of Paullinia species[5,6]. In view of the ethnopharmacological importance of the genus, this study was carried out with the aim of describing and analysing the anatomy and histochemistry of the leaves of three neotropical species of Paullinia, besides providing background data for pharmacological studies.
Materials and Methods
Adult leaves and distal leaflets of Paullinia micrantha Cambess. and P. trigonia Vell. were used from collections made in the state of Rio de Janeiro, in the following vegetation formations of the Atlantic Forest in the municipalities indicated: P. micrantha from the ombrophilous dense forest of Marambaia Island, Mangaratiba – G.V. Somner 824, 950 (RBR), and from the ombrophyllous dense forest of the Reserva Biológica da União, Rio das Ostras – G.V. Somner 1280 (RBR), P. trigonia Vell., from the sandy coastal plain of Marambaia Island, Mangaratiba – Somner et al. 1150 (RBR), and from the ombrophyllous dense forest of Itatiaia National Park, Itatiaia - Somner et al. 1317, 1449 (RBR). Distal leaflets of P. dasygonia Radlk. were obtained from segments taken from the herbarium specimens J.G. Wessels Boer 935 (NY) and T. Lasser in 1637 (NY), both from French Guiana. These leaflets were rehydrated in water with glycerin droplets, heated to 50 °C and then stored in 70% ethanol.
The leaflets were fixed in FAA (Formaldehyde, Alcohol, Acetic Acid, 10%:50%:5%+35% water) and stored in 70% ethanol.
For anatomical study, the leaflets were sectioned with a Ranvier microtome in the intercostal regions, the blade margin, the basal, central and apical thirds of the midrib, and the central region of the petiole and rachis, and then stained with astra blue/safranin solution and mounted in 50% glycerin[7].
Sections of newly collected specimens of P. micrantha and P. trigonia were used for the detection of different classes of chemicals and examination of the cell walls. This material had not been fixed and/or stored in 70% ethanol, and the sections were subjected to the following treatments: Sudan IV and Sudan Black B for lipids in general; 10% ferric chloride and 10% potassium dichromate to detect phenolic compounds; ruthenium red 0.02% for pectic substances; Fehling reagent for reducing sugars; aniline blue black, blue mercury bromophenol solution and Ponceau xylidine for detection of protein; lugol for starch; periodic acid/Schiff reagent (PAS) for neutral polysaccharides; Dragendorff reagent for detecting alkaloids; acetic acid and hydrochloric acid for identification of calcium oxalate crystals; sulfuric acid to identify saponins phloroglucinol hydrochloric acid to show lignified walls. Control treatments were applied to the histochemical tests following the indications of the authors cited above[7-10].
For examination of the epidermis, a dissociation technique was applied using hydrogen peroxide and glacial acetic acid (1: 1) at 60°C. The epidermis was stained with 1% safranin[7] and mounted in 50% glycerin.
For analysis of surface micromorphology, herborized leaflets segments were mounted on stubs and coated with a layer of gold. Photomicrographs were obtained using a scanning electron microscope, JEOL 5800 LV operating at 20KV.
The diagrams and photomicrographs were made using a microscope and an Olympus BX-51 with a capture system consisting of a Q color camera 5 and software Image-Pro Express. When necessary, the images were edited in Corel Photo-Paint ® 15 software and the plates were mounted using Corel DRAW® 15.
Results and Discussion
Leaf external morphology
The three species are lactiferous lianas, with two tendrils at the base of the leaf rachis (FIGURE 1A); the stem is lenticellate (FIGURE 1B). The leaves are alternate, imparipinnate compounds (FIGURE 1A), with triangular stipules (FIGURE 1C), marginate or winged rachis (FIGURE 1A), and hairy domatia between the main and secondary ribs on the abaxial surface (FIGURE 1D).
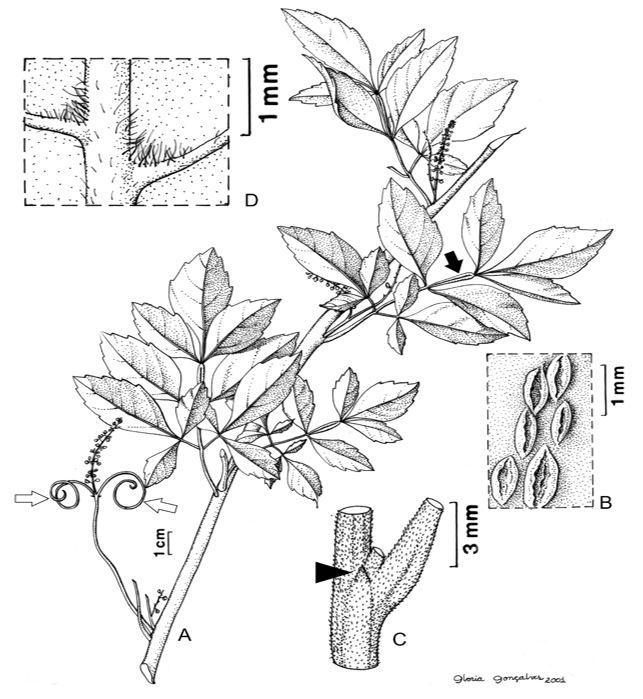
Anatomy
The leaves are hypostomatic (FIGURE 2A, C and E; 3A, C, E), with a dorsiventral mesophyll (FIGURE 4 A - D) and collateral vascular bundles (FIGURE 4C).
In front view, the cuticle of the epidermal cells ranges from striate (FIGURE 2B and D) to flat (FIGURE 3F), the anticlinal walls from undulate to straight on both adaxial (FIGURE 2A, C and E) and abaxial (FIGURE 3A, C and E) surfaces. Unicellular non glandular trichomes (FIGURE 2B, D and F; 3B and D) and multicellular glandular trichomes (FIGURE 3D and F) were observed on both sides.
It is noteworthy that there are variations in the shape, length and distribution of the trichomes. In P. dasygonia the non-glandular trichomes are short, straight (FIGURE 2 B) or curved (FIGURE 3B) and distributed in the intercostal region; long trichomes are observed in the region of the leaf ribs. In P. micrantha, short trichomes have a large base (FIGURE 2D), resembling an aculeus and occur in the intercostal region; on the leaf ribs there are also long trichomes. In P. trigonia the glandular trichomes vary from long to short (FIGURE 2F and 3E) and are distributed throughout the leaflet blade. In the leaf rib regions, there are rarely long trichomes.
The glandular trichomes are multicellular, stalked and capitate (FIGURE 3D and F). In P. dasygonia the stalk consists of two cells and the secretory head is formed by three to four cells. The variation in the number of head secretory cells leads to variation in the shape of the glandular trichomes. In P. micrantha both the peduncle and the head consist of four cells. In P. trigonia the stalk is formed by a single cell and the head by one to four cells (FIGURE 3F).
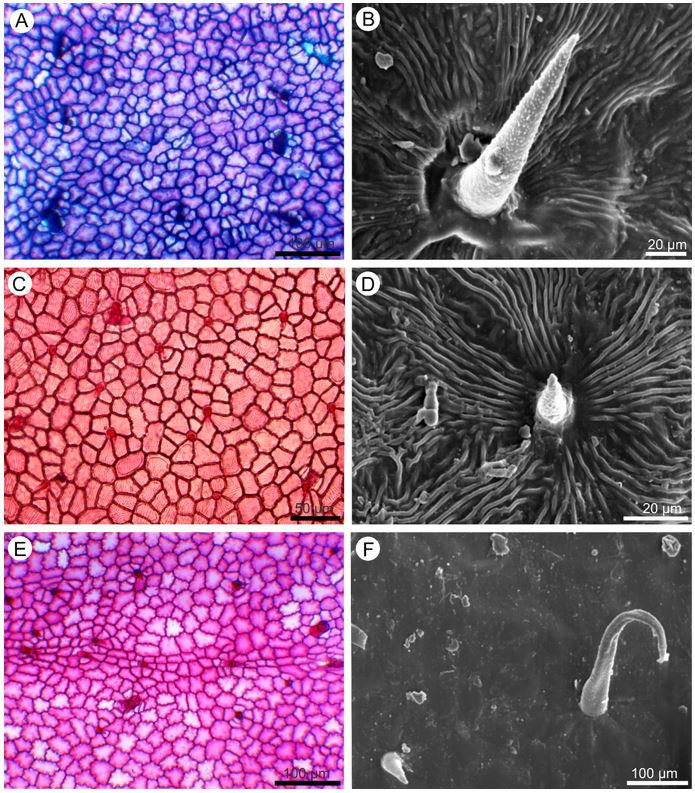
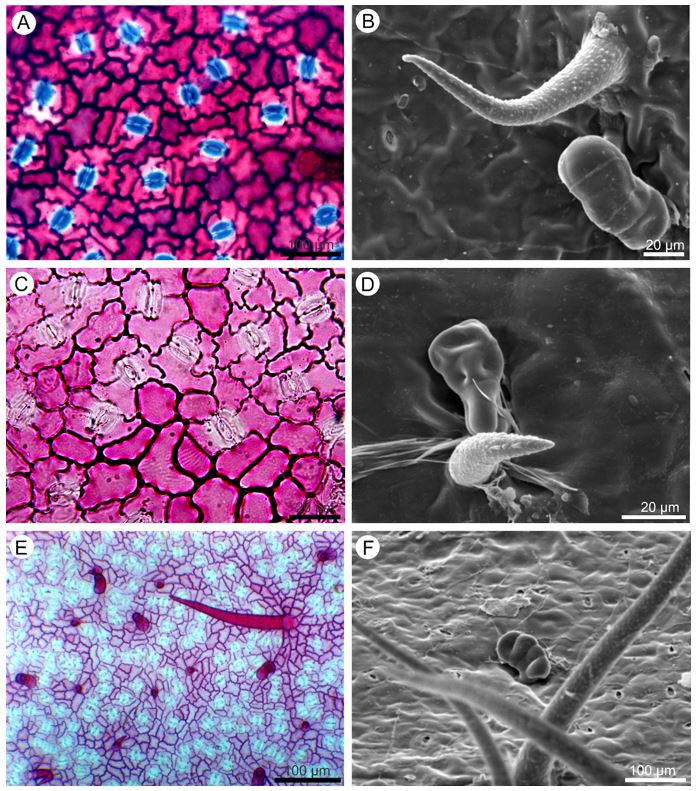
Transverse sections show that the epidermis on both surfaces of the leaflet blade is uniseriate and consists of cells with varying dimensions (FIGURE 4A, B and D). The cuticular layer is thickened on the adaxial side (FIGURE 4 B and D), and thin on the abaxial. On both sides of the leaflet blade, phenolic idioblasts are observed among the other epidermal cells (FIGURE 4B).
The mesophyll in the three species consists of palisade and spongy parenchyma (FIGURE 4A - D). The palisade parenchyma is composed of one layer of cells and the spongy parenchyma of approximately eight layers (FIGURE 4A and B). The vascular bundles are of collateral type, of different sizes, and are immersed in the spongy parenchyma (FIGURE 4B). In thicker vascular bundles, perivascular fibers are observed (FIGURE 4B).
The support tissue is formed from collenchyma and fibers. The collenchyma is of the annular type and occurs along the stem, on both sides of the midrib (FIGURE 4C) and in the leaflet margin. The fibers are present next to the phloem of the petiole and midrib (FIGURE 4C). The main vein vascular system of the three species is represented by two arches, consisting of collateral conductor bundles surrounded by fibers (FIGURE 4C).
Conspicuous laticifers in the mesophyll (FIGURE 4D) were observed in the three species, in the cortex of the midrib and petiole.
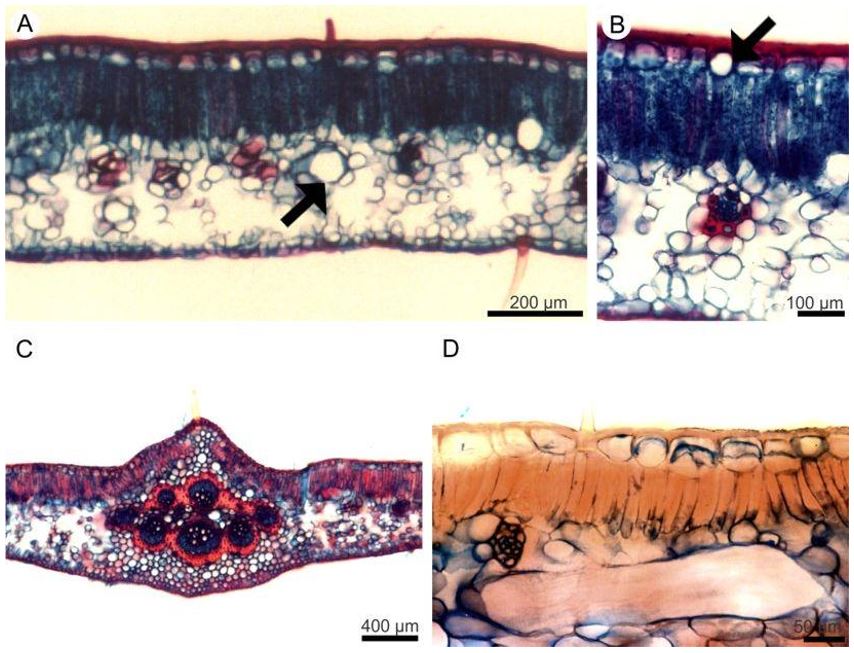
Histochemistry
The histochemical tests revealed lipid droplets, alkaloids, proteins and phenolic compounds present in the secretion in the lumen of the laticifers of both species; thick cuticle on the adaxial surface and thin cuticle on the abaxial face of the epidermis of the leaflet blade of P. micrantha and P. trigonia; phenolic compounds in numerous idioblasts distributed throughout the petiole and leaflet blade of both species; neutral polysaccharides and phenolic compounds in the protoplast of the secretory trichome cells of both species; mucilage consisting of acidic and neutral polysaccharides, and phenolic substances in epidermal idioblasts of the leaflet blade on the adaxial surface of both species; starch grains in petiole parenchyma cells, the main vein and palisade tissue of both species. Saponins were identified in the trichomes, and in general in the mesophyll and midrib. The phenolic compounds reacted with potassium dichromate and ferric chloride, in addition to reacting to periodic acid/Schiff reagent (PAS) acquiring an intense pink color.
The studied species have very similar leaf morphology, which causes difficulties in their identification. However, leaf morphoanatomical differences were observed that are important for distinguishing the species.
The anatomical characters described for the three studied species, such as hypostomatic leaves, non-glandular and glandular trichomes, idioblasts, laticifers, dorsiventral mesophyll and conductor bundles of collateral type are in agreement with those recorded by[11], for several species of the Sapindaceae. We can infer that these anatomical features are genetically determined and are good character for species recognition in this family, including fragmented material, even when in powder form.
The histochemical tests carried out in this study detected the presence of several classes of chemicals such as acids and neutral polysaccharides, lipids, phenolic compounds, alkaloids and proteins. These substances were detected in all regions of the leaf in different morphological types of secretory structures.
Laticifers were observed in flowers of Paullinia alata G. Don, P. clavigera Schltdl., P. dasystachya Radlk., P. obovata (Ruiz & Pav.) Pers. and P. pachycarpa Benth.by[12]. The presence of a latex-like substance produced in secretory cells of irregular shape which in the leaves appear as transparent points[11]. The structures described by the authors cited above are here interpreted as laticifers. The study of structural, developmental and chemical features of laticifers of 67 species of Sapindaceae, among them 10 species of Paullinia, stated that the occurrence of laticifers in this family is broader than previously reported, and suggest that the characters of laticifers may be useful in understanding the generic relationships within the family[13].
The latex consists of a wide variety of biologically active chemical substances, among which we highlight the terpenoids, lipids, starch grains, alkaloids, polysaccharides, phenolic substances, sugars and proteins[14-16]. In the secretion produced by laticifers in Paullinia trigonia and P. micrantha, alkaloids, lipids, proteins and phenolic substances have been identified.
The essential oils and alkaloids, in low concentrations, can function as an insect repellent or be highly toxic to animals, providing defence against herbivores. Lipophilic secretions, especially terpenes, are usually reported as having a role in the chemical defense of plants, working as a protection against herbivores and pathogens and can serve as an energy source and/or reserve, and may also attract or repel animals[17,18].
Alkaloids and phenolic compounds are considered to be substances which deter herbivores. Alkaloids can function as temporary reserve products for protein synthesis[4] and as agents that protect plant organs, due to their high toxicity to phytophagous insects. Alkaloid-bearing plants are less palatable because of their more difficult digestion and bitter taste[19]. Such substances are frequently found in vacuoles or as salts in the cell walls and their concentration may vary during the year or be restricted to the plant organs at different annual seasons[20]. Allelopathic action, antimicrobial and antifungal effects and toxicity in insects and molluscs have been attributed to alkaloids[21] as well as laxative, emetic, cough sedation, antigout, antitumor, antimalarial, antispasmodic, stimulant and depressant pharmacological properties and hallucinogenic effects on the central nervous system[22,23].
Proteins found in the latex are possibly associated with defence mechanisms against microorganisms, but further studies are needed to determine the function of proteins in plant protection[24].
The presence of phenolic compounds is referred to as a group of substances of great importance in protection against herbivores, microorganisms, excess ultraviolet radiation and also to help the cell protoplast to maintain its integrity when subjected to water stress[25]. Pharmacologically, phenolic substances have astringent, healing, antiseptic, antioxidant, vasoconstrictor, hemostatic and anti-inflammatory properties[23,26] it is believed that there are other functions related to phenolic substances, although there are still doubts about the totality of its functions. Besides being present in secretory structures, production sites and secretion of secondary metabolites, phenolic compounds and alkaloids were also recorded in unspecialized cells such as parenchyma.
Mucilage is a secretion, consisting mainly of a mixture of acidic and/or neutral heteropolysaccharides, proteins and phenolic substances. It is widely distributed in plants, forming colloidal solutions that become viscous in contact with water[27]. These substances can perform different functions in plants, including the protection of developing structures and organs, water retention, carbohydrate reserves, reduction of transpiration, protection against radiation by scattering or reflecting incident light, protection against herbivory, lubricating root apices, insect capture in insectivorous plants, as an adhesive in seed dispersal and in the regulation of seed germination[10,27].
Secretory trichomes and mucilaginous cells were cited by[11] for the Sapindaceae. The mucilage present in the secretory trichomes and idioblasts of the studied species is composed of acidic and neutral polysaccharides and phenolic substances. suggests that in Althaea officinalis L. different fractions of mucilage have different functions[27]. Water storage is carried out by the acid fraction, which has its production peak in the summer months and the storage of carbohydrates is done by the neutral fraction, which has its maximum production during the winter. The presence of phenolic compounds in the mucilage, mainly tannins, is of antimicrobial importance and also in protection against herbivores, and is an important chemical protective barrier[10,27]. The presence of phenolic substances identified in the different morphological types of secretory structures in the studied species, can perform the functions attributed by the above-mentioned authors.
The main function of the mucilage produced by the trichomes of the studied species is probably to lubricate and protect organs against desiccation, as reported for secretory trichomes in Nymphoides peltata (S. G. Gmel.) Kuntze[28].
Despite the fact that the ontogenetic origin of mucilage idioblasts has not been investigated, we observed that such cells in adult organs have much larger dimensions than the adjacent cells. Mucilaginous cells, in their young state, do not differ from neighboring parenchymal cells, becoming much larger as they mature, and this characteristic was also observed in the studied species[14].
The saponin detected in the histochemical tests is a common feature in the Sapindaceae. The use of Sapindaceae as fish poisons, human poisons and arrowheads poisons is attributed to this class of terpenes[4].
Conclusion
In the different morphological types of secretory structures of the species of Paullinia studied here, we recorded a diversity of classes of chemical substances that justify the use of the species of the genus as medicinal. These chemical compounds can be related with the plant protection against desiccation and within the plant act in water and carbohydrate storage, affecting the water balance, in resistance to desiccation and as deterrents to herbivores and pathogens as alkaloids and phenolic compounds present in the latex. We consider that reports of the use of other species of the genus for medicinal purposes and the occurrence of different kinds of bioactive substances in P. dasygonia, P. micrantha and P. trigonia are sufficiently strong indicators to justify the pharmacological investigation of these three species and others in the genus.
Financing source
None.
Conflict of interests
There is no conflict of interests.
Acknowledgments
The authors thank Glória Gonçalves for the line drawings, and the Curator of the New York Botanical Garden Herbarium (NY) for providing botanical material used for the anatomical study. The authors also thank Simon Joseph Mayo from Royal Botanical Gardens Kew by the revision of the manuscript.
Contribution
Study design: GVS.
Data curation: GVS; RRP: JFR; RCOA.
Data collection: GVS; RRP: JFR; RCOA.
Data analysis: RRP: JFR; RCOA.
Writing of the original manuscript: GVS; RRP: JFR; RCOA.
Writing the review and editing: GVS; RRP; JFR.
References
1. Chery JG, Acevedo-Rodríguez P, Rothfels CJ, Chelsea D, Specht CD. Phylogeny of Paullinia L. (Paullinieae: Sapindaceae), a diverse genus of lianas with dynamic fruit evolution. Mol Phylogenet Evol. 2019; 140: 1-12. [https://doi.org/10.1016/j.ympev.2019.106577].
2. Somner GV, Medeiros H. Paullinia in Flora do Brasil. Jardim Botânico do Rio de Janeiro. 2020; [accessed 03 set. 2021]. Available in: [http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB20914].
3. Basile A, Ferrara L, Del Pezzo M, Mele G, Sorbo S, Bassi P et al. Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana Mart. J Ethnopharmacol. 2005; 102: 32–36. [https://doi.org/10.1016/j.jep.2005.05.038].
4. Beck HT. A survey of the useful species of Paullinia. Adv Econ Bot. 1990; 8: 41–56.
5. Radlkofer L. Monographie der Sapindaceen-Gattung Paullinia. K. B. Munich: Akademie; 1895. [https://doi.org/10.5962/bhl.title.65993].
6. Lindorf H. Anatomía foliar de especies de un bosque húmedo en el Territorio Federal Amazonas. Mem Soc Cienc Nat. La Salle. 1992; 52: 65-91.
7. Kraus JE, Arduin M. Manual básico de métodos em morfologia vegetal. Seropédica: EDUR; 1997.
8. Johansen DA. Plant microtechinique. New York: MacGraw-Hill; 1940.
9. Costa AF. Farmacognosia. 2ª ed. Lisboa: Fundação Calouste Gulbekian; 1982.
10. Pimentel RR, Machado SR, Rocha JF. Estruturas secretoras de Pavonia alnifolia (Malvaceae), uma espécie ameaçada de extinção. Rodriguésia. 2011; 62(2): 253-262. [https://doi.org/10.1590/2175-7860201162203].
11. Metcalfe CR, Chalk L. Anatomy of dicotyledons. v.1. Oxford: Clarendon Press; 1950.
12. Weckerle CS, Rutishauser R. Gynoecium, fruit and seed structure of Paullinieae (Sapindaceae). Bot J Linn Soc. 2005; 147: 159–189.
13. Medina MC, Sousa-Baena MS, Prado E, Acevedo-Rodríguez P, Dias P, Demarco D. Laticifers in Sapindaceae: structure, evolution and phylogenetic importance. Front Plant Sci. 2021; 11: 612985. [https://doi.org/10.3389/fpls.2020.612985].
14. Fahn A. Secretory tissues in plants. London: Academic Press; 1979.
15. Appezzato-Da-Glória B, Estelita MEM. Laticifer systems in Mandevilla illustris and M. velutina Apocynaceae. Acta Soc Bot Pol. 1997; 66 (3-4): 301-305. https://doi.org/10.5586/asbp.1997.035
16. Rabelo GR, Marques JBC, Zottich U, Dias GB, Miguel EC, Gomes VM et al. Leaf structure, microanalysis and characterization of the látex protein profile of Pachystroma longifolium (Nees) I.M. Jonhst. (Euphorbiaceae) in a seasonally dry Atlantic Forest. Acta Bot Bras. 2011; 25(1):150-159. [https://doi.org/10.1590/S0102-33062011000100018].
17. Werker E, Fahn A. Secretory Hairs of Inula viscosa (L.) Ait. Development, Ultrastructure, and Secretion. Bot Gaz. 1981; 142: 461-476.
18. Rodriguez E, Healey PL, Mehta I. Biology and chemistry of plant trichomes. New York: Plenum; 1984.
19. Henriques ATH, Kerbe VA, Moreno PRH. Alcaloides: generalidades e aspectos básicos. In: Simões, CMO et al. (org.) Farmacognosia da planta ao medicamento. 2ª ed. Porto Alegre e Florianópolis: Editora da UFRGS e Editora da UFSC; 2002. p. 641-656.
20. Martins ER, Castro DM, Castellani DC, Dias JE. Plantas medicinais. Viçosa: Ed. UFV; 1995.
21. Medeiros ARM. Alelopatia: importância e suas aplicações. Horti Sul. 1990; 1(3):27-32.
22. Martín JJ, Moll MCN, Zurita AZ. Alcaloides. In:Fresno AM (ed.). Farmacognosia General. Madri: Ed. Síntesis; 1999. p. 251-262.
23. Cunha AP. Farmacognosia e Fitoquímica. Lisboa: Fundação Calouste Guilbenkian; 2005.
24. Klein DE, Gomes VM, Silva-Neto SJ, Da Cunha M. The structure of colleters in several species of Simira (Rubiaceae). Ann Bot. 2004; 94: 733-740. [https://doi:10.1093/aob/mch198].
25. Paiva EAS, Machado SR. The Floral Nectary of Hymenaea stigonocarpa (Fabaceae, Caesalpinioideae): Structural Aspects During Floral Development. Ann Bot. 2008; 101: 125-133. [https://doi:10.1093/aob/mcm268].
26. Raphael KR, Kuttan R. Inhibition of experimental gastric lesion and inflammation by Phyllanthus amarus extract. J Ethnopharmacol. 2003; 87(2-3): 193-197. [http://doi:10.1016/s0378-8741(03)00120-x].
27. Gregory M, Baas P. A survey of mucilage cells in vegetative organs of the dicotyledons. Isr J Bot. 1989; 38: 125-174.
28. Meyberg M. Cytochemistry and ultrastructure of the mucilage secreting trichomes of Nymphoides peltata (Menyanthaceae). Ann Bot. 1988; 62: 537-547. [https://doi.org/10.1093/oxfordjournals.aob.a087690].