ARTIGO DE PESQUISA
Identification of larvicidal activity of medicinal plants against Aedes aegypti (Diptera: Culicidae)
Abstract
Aedes aegypti is the main transmitter of several arboviruses, such as dengue, the world's most widespread arbovirus. The main method of controlling arbovirus transmission is through vector control, using insecticides. But its continuous, cases of resistance occur. Thus, research is seeking new botanical insecticide alternatives, because have multiple active compounds can be applied on the vector, contributing to the control of arbovirus transmission. The aim of this study is to identify plants cultivated in the Western Amazon, which have larvicidal activity against A. aegypti. Ethanolic extracts from ten plants were obtained to verify the larvicidal biopotential against third instar larvae of A. aegypti. The promising ethanolic extracts was Fridericia chica, which was submitted to liquid-liquid partition for larvicidal activity evaluation and phytochemical analysis. Among the partitions analyzed, the aqueous partition of F. chica showed larvicidal biopotential against A. aegypti, being the first report in the literature of its larvicidal activity. In the phytochemical prospection of the aqueous partition of F. chica, we observed the presence of catechins, condensed tannins and the flavone group, flavonols and xanthones. These groups of metabolites have been reported in the literature as larvicidal agents.
- Keywords:
- Aedes.
- Plants.
- Insecticides.
- Biochemistry.
- Arbovirus infections.
Introduction
The Aedes aegypti mosquito stands out for being the transmitter of arboviruses, such as dengue, chikungunya, yellow fever, zika[1–3], where dengue is the most widespread disease globally in comparison to other arboviruses[4]. A. aegypti is a vector with high efficiency in arbovirus transmission[5] . Study shows that mosquitoes can transmit at the same time, to a vertebrate, more than one arbovirus, which makes this fact worrisome, since about 75.2% of the countries are suitable for the development of A. aegypti[4,6].
Arboviruses whose vector is A. aegypti can cause mild illness to and death[7]. It may affect neurological syndromes. In 2015, there was an outbreak of Zika in Brazil, in which newborns, from mothers infected by the virus in the first trimester, presented microcephaly[8,9]. Some cases, of Guillain Barré syndrome in infected adults[10,11]. While, Chikungunya virus infections is capable of afflicting polyarthralgia and arthritis, even after cure, it may persist these symptoms for months to years[12,13].
For the control of arbovirus transmission, studies have been carried out to produce vaccines. However, there are certain limitations, such as the efficiency in immune responses against the four dengue serotypes is still an issue[14,15]. Therefore, the main method for controlling arbovirus transmission is to prevent the development and spread of the vector, by applying synthetic insecticides against larvae and mosquitoes[16,17]. Although, with the continuous use of insecticides, cases of resistance have been reported[18–20].
Thus, to reduce the incidence of these diseases, the epidemiological surveillance has applied synthetic insecticides to control the vector, but with their continuous use cases of resistance have arisen[21,22]. For this reason, many researches are looking for control alternatives for A. aegypti by means of plant metabolites[23–25]. Since botanical insecticides can be composed of multiple active compounds, which makes the emergence of resistance difficult[26], in addition to being biodegradable and specific for the target species[27].
Studies have shown the effectiveness of several plant species cultivated in the Amazon against A. aegypti larvae, such as Tapura amazonica, Piper aduncum, Piper tuberculatum, Simaba polyphylla and Acmella oleracea[25,28], that can be an alternative for mosquito control, in order to interrupt the development phase and, consequently, the transmission of arbovirus. In some cases, plants are known to have larvicidal activity against A. aegypti, such as Tagetes patula, our positive control in the larvicidal bioassays. Thus, the objective of this work is to identify plants species cultivated in the Western Amazon that have insecticidal activity against A. aegypti.
Material and Methods
Obtaining plant material
Ten plants used in popular medicine and located in Acre state, were collected in the mornings of May to September between the years 2017 to 2019. The plant materials were identified used morphological identification keys of each family, incubated for in an oven at 40ºC until brittle (on average, 3 days drying time) and triturated. Then, the technique of exhaustive maceration was adopted using 99% ethanol, to be later filtered, evaporated using a Quimis® rotary evaporator at a temperature of 55ºC and lyophilized, to obtain the ethanolic extracts (TABLE 1).
| Scientific name | Family | Used part | Popular name | Exsiccate number |
|---|---|---|---|---|
| Alternanthera brasiliana | Amaranthaceae | Leaf | Penicilin | UFACPZ: 20740 |
| Kalanchoe pinnata | Crassulaceae | Leaf | Leaf of fortune | UFACPZ: 20741 |
| Senna alata | Fabaceae | Leaf | Sickle pod | UFACPZ: 20739 |
| Euphorbia tirucalli | Euphorbiaceae | Aerial part | Pencil cactos | UFACPZ20440 |
| Fridericia chica | Bignoniaceae | Leaf | Cricket wine | UFACPZ: 20562 |
| Jatropha gossypiifolia | Euphorbiaceae | Leaf | Bellyache bush | UFACPZ20558 |
| Momordica charantia | Cucurbitaceae | Leaf | Momordica | UFACPZ20561 |
| Morinda citrifolia | Rubiaceae | Leaf | Noni | UFACPZ20557 |
| Plectranthus barbatus | Lamiaceae | Leaf | Coleus barbatus | UFACPZ20560 |
| Tagetes patula | Asteraceae | Aerial part | Mexican marigold | UFACPZ20445 |
The ethanolic extracts that manifested larvicidal activity were submitted to liquid-liquid partition, using the solvents hexane, dichloromethane and ethyl acetate, where the initial solution used was methanol and distilled water (1:10).
Obtaining A. aegypti larvae
To obtain A. aegypti larvae, it was necessary to collect eggs of the species near residences located in ten selected neighborhoods of Rio Branco, Acre: Loteamento Joafra, Mocinha Magalhães, Cruzeirinho, Santa Helena, Santa Cecília, Universitario, Vila Betel, Conjunto Esperança, Laélia Alcântara and Rui Lino.
The method adopted for collection was by means of ovitramps, containing a 0.04% brewer's yeast-based attractant solution and a eucatex palette[29]. The ovitramps were installed for ten days, in the peridome, with the solution and palette changed on the fifth day of installation.
After this period, the pallets were taken to the Intermediate Phytotherapeutic Laboratory of the Federal University of Acre (UFAC) for identification and counting of A. aegypti eggs, where these eggs were stored in a closed styrofoam box for a maximum period of two months until hatching for the creation and maintenance of the F0 generation. Subsequently, the pallets containing A. aegypti eggs were placed in a container with distilled water for hatching, for subsequent identification of the larvae by the identification keys described by Consoli and Oliveira[30].
The larvae were fed with fish food (Alcon Basic®) until they reached the pupal stage and then transferred to cages for mosquito breeding to obtain the F1 generation. The mosquitoes were fed with absorbent cotton dampened with 10% sucrose solution[30] and artificial feeder Glytube[31]. The F1 generation of eggs were stored to perform larvicidal activity assays.
Larvicide test
The larvicide test followed the recommendations of the World Health Organization[32] and Consoli and Oliveira[30] with adaptations. The ethanolic extracts were solubilized in dimethyl sulfoxide (DMSO) at a concentration of 250 mg/mL.
To evaluate the larvicidal biopotential of the extracts against A. aegypti, initial screening was performed with 100 ppm of the ethanolic extracts in 10mL of distilled water and in the presence of 20 third instar larvae. As a positive control, we used the ethanolic extract of Tagetes patula[33,34], described in the literature with larvicidal activity, and as a negative control, 0.04% DMSO. The mortality rate was recorded at 24 and 48 hours. For analysis, four repetitions were performed.
Subsequently, the promising plants with larvicidal activity, their partionates were obtained, lyophilized and solubilized in 125 mg/mL using DMSO. The partionates were submitted to larvicidal test under the same conditions as the initial screening, and 300 ppm of each partition was used.
After performing three replicates, the data was analyzed in the statistical program GraphPad Prism version 8.0.1, where means and standard deviation were calculated. As well as being used to perform ANOVA and t-tests.
Phytochemical prospection
The phytochemical study followed the protocol described by Matos [35]. The following classes were analyzed: tannins, phenols, saponins, reducing sugars, leucoanthocyanidins, catechins, flavanones, anthocyanidins, and flavonoids.
Results and Discussion
Among the ten extracts analyzed, only three plants extracts showed larvicidal activity against A. aegypti at the concentration 100 ppm, those that showed the best result was F. chica (36.2% of mortality), while the positive control (T. patula) was 48.7% larvae mortality up to 48 hours. While P. barbatus and S. alata showed larvicidal activity of less than 25% (FIGURE 1). However, larvicidal activity was observed at 24 hours in ethanolic extracts of F. chica (32,5% of mortality), and few deaths (3,7%) at 48 hours in your four replicates.
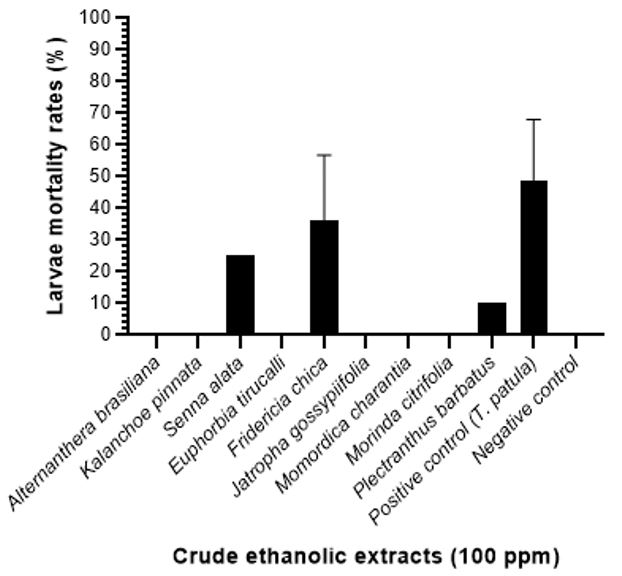
Therefore, the ethanolic extract of F. chica was partitioned into hexane, dichloromethane, ethyl acetate and aqueous fractions, and all were tested at 300 ppm against F1 generation larvae of A. aegypti. All partitions showed mortality of larvae up to 48 hours and in that there are differences in the performance of the larvicidal activity of partition (p-value = 0,0151). However, when analyzed individually, only the aqueous partition of F. chica (p-value = 0,0114) showed larvicidal activity when compared to the negative control (FIGURE 2).
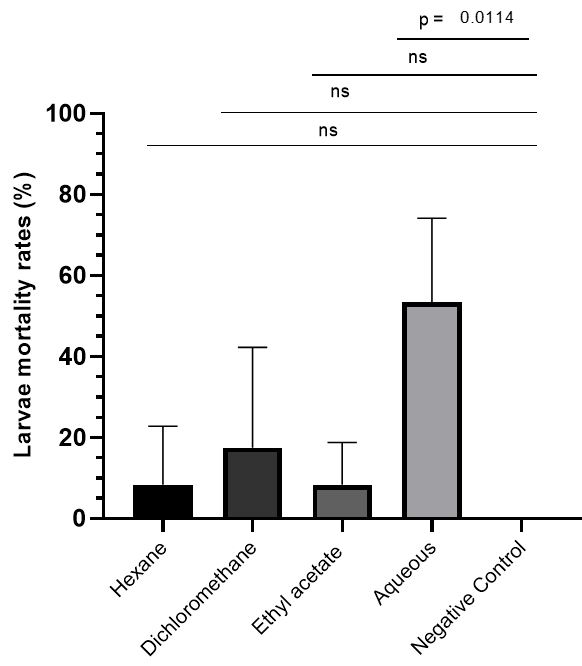
The mortality rates of larvae were analyzed by the One-way Anova statistical method, resulting that the performance in larvicidal action depends on the partition analyzed (p-value=0.0151). Only the aqueous partition exhibits larvicidal activity when compared to the negative control when analyzed by t test.
After these results, the phytochemical prospection of the partitions of F. chica was conducted. The metabolites detected in the aqueous partition of F. chica were condensed tannins, catechins and the flavone, flavonol and xanthone groups (TABLE 2). The phytochemical prospection of our positive control was not analyzed due to the low yield of its extract to be submitted to liquid-liquid partition.
| Metabolites | H | D | AC | AQ |
|---|---|---|---|---|
| Phenols | - | + | - | - |
| Flavones, flavonols and xanthones | - | IR | - | + |
| Chalcones and aurones | - | - | + | IR |
| Flavanonols | + | - | IR | IR |
| Cathechins | - | IR | - | + |
| Condensed tannins | + | IR | + | + |
| H: hexane partition; D: dichloromethane partition; AC: ethyl acetate partition; AQ: aqueous partition; IR: inconclusive result; -: negative, +: positive. Phytochemical prospection negative to anthocyanins, anthocyanidins, leucoanthocyanidins, flavanones and hydrolyzable tannins. | ||||
In our studies, the ethanolic extract of T. patula, the positive control, the mortality rate reached 48.7% in 48 hours at the concentration of 100 ppm. The species T. patula is described in the literature manifesting larvicidal activity against the genus Aedes, as reported its activity against larvae of A. fluviatilis and A. aegypti, whose mortality rate was 65.6% at a concentration of 100 mg/L in 24 hours and 92% at a concentration of 153.6 mg/L in 48 hours, respectively[33,34].
The plants that demonstrated low or no activity in this research, such as Euphorbia tirucalli[36] and Plectranthus barbatus[37] should be further explored, since studies indicate a potential larvicidal agent against A. aegypti. This fact may be related to factors such as: extraction procedure, solvent used, place of collection and other factors that may cause divergence of larvicidal activity and the detection of classes of metabolites[38].
The species F. chica is known for several pharmacological activities, such as antimicrobial[39] and anti-inflammatory[40]. And according to PubMed, Wiley Online Library, Scielo and Science Direct databases, this is the first report in the literature of the crude extract of F. chica with larvicidal activity against A. aegypti. However, recent research has shown that its essential oil demonstrated insecticidal activity on larval and pupal stages of A. aegypti, moreover, mosquitoes from the pupae that survived[41]. Thus, the phytochemical prospection of F. chica, we can observe the presence of catechins, condensed tannins and the group of flavones, flavonols and xanthones.
Comparing the constituents detected in the promising partitions of F. chica, there are investigations that demonstrate that these metabolites present insecticidal activity, such as condensed tannins, flavones and catechins extracted from several plant species against A. aegypti[42–44]. There is even a study that points out that there are morphological changes in larvae that were exposed to insecticide metabolites, such as catechins, caused lesions in the midgut epithelium and deformation of the anal papillae[44].
Conclusion
Therefore, among the ten plants analyzed, Fridericia chica showed the best larvicidal biopotential against A. aegypti. Among these plants, this is the first report of F. chica extract showing larvicidal activity, and it may be a potential alternative agent in controlling the proliferation of the A. aegypti. However, more studies are needed to configure it as an alternative tool for the control of A. aegypti. Mainly, with the increasing reports of resistance to chemical insecticides usually used in urban environments.
Funding
This research received no external funding.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgment
The Federal University of Acre and the Municipal Health Secretary. This work was carried out with the support of the Coordination for the Improvement of Higher Education Personnel - Brazil (CAPES) - Funding Code 001.
Contributors
Concepção do estudo: ECNS; ECBC; RCR.
Curadoria dos dados: ECNS; TLA; AML; ECBC.
Coleta de dados: ECNS; TLA; AML; MAOS; PBR; GAB; JAF; JLS; PJPM.
Análise dos dados: ECNS; ECBC; TLA; AML.
Redação do manuscrito original: ECNS; ECBC.
Redação da revisão e edição: ECNS; ECBC
Referências
1. Rufino BP, Santos ECN, Maggi EL, Costa ECB. Mayaro Virus: an Emerging Arbovirosis in Brazil? Multidiscip Sci Reports. 2022; 2: 1–24. [https://doi.org/10.54038/ms.v2i2.19].
2. Souza-Neto JA, Powell JR, Bonizzoni M. Aedes aegypti vector competence studies: a review. Infect Genet Evol. 2019; 67: 191–209. [https://doi.org/10.1016/j.meegid.2018.11.009].
3. Santos ECN, Barbosa GA, Rufino PB, Lima AA, Araújo TL, Costa ECB. Zika Vírus: dos aspectos epidemiológicos ao tratamento. In: Saúde da criança e adolescente: epidemiologia, doenças infecciosas e parasitárias. 2019. p. 88–106. [https://doi.org/10.35170/ss.ed.9786580261109.06].
4. Leta S, Beyene TJ, Clercq EM De, Amenu K, Revie CW, Kraemer MUG. Global risk mapping for major diseases transmitted by Aedes aegypti and Aedes albopictus. Int J Infect Dis. 2018; 67: 25–35. [https://doi.org/10.1016/j.ijid.2017.11.026].
5. Patterson J, Sammon M, Garg M. Dengue, Zika and Chikungunya: emerging arboviruses in the new world. West J Emerg Med. 2016; 17: 671–9. [https://doi.org/10.5811/westjem.2016.9.30904].
6. Rückert C, Weger-Lucarelli J, Garcia-Luna SM, Young MC, Byas AD, Murrieta RA et al. Impact of simultaneous exposure to arboviruses on infection and transmission by Aedes aegypti mosquitoes. Nat Commun. 2017; 8. [https://doi.org/10.1038/ncomms15412].
7. Siqueira JB, Martelli CMT, Coelho GE, Simplício ACR, Hatch DL. Dengue and dengue hemorrhagic fever, Brazil, 1981-2002. Emerg Infect Dis. 2005; 11: 48–53. [https://doi.org/10.3201/eid1101.031091].
8. Mlakar J, Korva M, Tul N, Popović M, Poljšak-Prijatelj M, Mraz J et al. Zika Virus Associated with Microcephaly. N Engl J Med. 2016; 374: 951–8. [https://doi.org/10.1056/NEJMoa1600651].
9. Schuler-Faccini L, Ribeiro EM, Feitosa IML, Horovitz DDG, Cavalcanti DP, Pessoa A et al. Possible Association Between Zika Virus Infection and Microcephaly — Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016; 65: 1–4. [https://doi.org/10.15585/mmwr.mm6503e2er].
10. Brasil P, Sequeira PC, Freitas ADA, Zogbi HE, Calvet GA, Souza RV et al. Guillain-Barré syndrome associated with Zika virus infection. Lancet [Internet]. 2016; 387: 1482. Available from: [https://doi.org/10.1016/S0140-6736(16)30058-7].
11. Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: A case-control study. Lancet. 2016; 387: 1531–9. [https://doi.org/10.1016/S0140-6736(16)00562-6].
12. Sales GMPG, Barbosa ICP, Neta LMSC, Melo PL, Leitão RA, Melo HMA. Treatment of Chikungunya chronic arthritis: a systematic review. Rev Assoc Med Bras. 2018; 64: 63–70. [https://doi.org/10.1590/1806-9282.64.01.63].
13. Schilte C, Staikovsky F, Couderc T, Madec Y, Carpentier F, Kassab S et al. Chikungunya Virus-associated Long-term Arthralgia: A 36-month Prospective Longitudinal Study. PLoS Negl Trop Dis. 2013; 7 (3):e2137. [https://doi.org/10.1371/journal.pntd.0002137] [https://pubmed.ncbi.nlm.nih.gov/23556021/] .
14. Yang Y, Meng Y, Halloran ME, Longini IM. Dependency of Vaccine Efficacy on Preexposure and Age: A Closer Look at a Tetravalent Dengue Vaccine. Clin Infect Dis. 2018; 66: 178–84. [https://doi.org/10.1093/cid/cix766].
15. White LJ, Young EF, Stoops MJ, Henein SR, Adams EC, Baric RS et al. Defining levels of dengue virus serotype-specific neutralizing antibodies induced by a live attenuated tetravalent dengue vaccine (Tak-003). PLoS Negl Trop Dis [Internet]. 2021; 15: 1–15. Available from: [http://dx.doi.org/10.1371/journal.pntd.0009258].
16. Carvalho FD, Moreira LA. Why is Aedes aegypti Linnaeus so Successful as a Species? Neotrop Entomol. 2017; 46: 243–55.[https://doi.org/10.1007/s13744-017-0520-4].
17. Rodríguez MM, Bisset JA, Fernández D. Levels of insecticide resistance and resistance mechanisms in Aedes aegypti from some Latin American countries. J Am Mosq Control Assoc. 2007; 23: 420–9. [https://doi.org/10.2987/5588.1].
18. Carvalho MSL, Caldas ED, Degallier N, Vilarinhos PTR, Souza LCKR, Yoshizawa MAC et al. Susceptibility of Aedes aegypti larvae to the insecticide temephos in the Federal District, Brazil. Rev Saude Pública. 2004; 38: 623–9. [https://doi.org/10.1590/S0034-89102004000500002].
19. Da-Cunha MP, Lima JBP, Brogdon WG, Moya GE, Valle D. Monitoring of resistance to the pyrethroid cypermethrin in Brazilian Aedes aegypti (Diptera Culicid. Mem Inst Oswaldo Cruz. 2005; 100: 441–4. [https://doi.org/10.1590/S0074-02762005000400017].
20. Braga IA, Lima JBP, Soares SS, Valle D. Aedes aegypti resistance to temephos during 2001 in several municipalities in the states of Rio de Janeiro, Sergipe, and Alagoas, Brazil. Mem Inst Oswaldo Cruz. 2004; 99: 199–203. [https://doi.org/10.1590/S0074-02762004000200015].
21. Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS Negl Trop Dis. 2017; 11: e0005625. [https://doi.org/10.1371/journal.pntd.0005625].
22. De Araújo AP, Paiva MHS, Cabral AM, Cavalcanti AEHD, Pessoa LFF, Diniz DFA et al. Screening Aedes aegypti (Diptera: Culicidae) Populations from Pernambuco, Brazil for Resistance to Temephos, Diflubenzuron, and Cypermethrin and Characterization of Potential Resistance Mechanisms. J Insect Sci. 2019; 19: 1–15. [https://doi.org/10.1093/jisesa/iez054].
23. Ciccia G, Coussio J, Mongelli E. Insecticidal activity against Aedes aegypti larvae of some medicinal South American plants. J Ethnopharmacol. 2000; 72: 185–9. [https://doi.org/10.1016/S0378-8741(00)00241-5].
24. Pohlit AM, Quinard ELJ, Nunomura SM, Tadei WP, Hidalgo AF, Pinto ACS et al. Screening of plants found in the State of Amazonas, Brazil for activity against Aedes aegypti larvae. Acta Amaz. 2004; 34: 97–105. [https://doi.org/10.1590/S0044-59672004000100012].
25. Omena MC, Navarro DMAF, Paula JE, Luna JS, Lima MRF, Sant&'Ana AEG. Larvicidal activities against Aedes aegypti of some Brazilian medicinal plants. Bioresour Technol. 2007; 98(13): 2549–56. [https://doi.org/10.1016/j.biortech.2006.09.040].
26. Ghosh A, Chowdhury N, Chandra G. Plant extracts as potential mosquito larvicides. Indian J Med Res. 2012; 135(5): 581–98. PMCID: PMC3401688. PMID: 22771587. [https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3401688/].
27. Rodrigues AM, Sampaio CG, Souza JSN, Campos AR, Silva ABR, Morais SM et al. Different susceptibilities of Aedes aegypti and Aedes albopictus larvae to plant-derived products. Rev Soc Bras Med Trop. 2019; 52. [https://doi.org/10.1590/0037-8682-0197-2018].
28. Araújo IF, Araújo PHF, Ferreira RMA, Sena IDS, Lima AL, Carvalho JCT et al. Larvicidal effect of hidroethanolic extract from the leaves of Acmella oleracea L. R. K. Jansen in Aedes aegypti and Culex quinquefasciatus. South African J Bot. 2018; 117: 134–40. [https://doi.org/10.1016/j.sajb.2018.05.008].
29. Anunciação SCM. Ovitrampa sem acúmulo de água: metodologia segura para coleta de ovos de Aedes? Rio de Janeiro, 2017. 25 f. Trabalho de Conclusão de Curso, Especialização em Entomologia Médica [Programa de Pós-Graduação em Entomologia Médica] - Instituto Oswaldo Cruz. Fundação Oswaldo Cruz. Rio de Janeiro, RJ, Brasil. 2017. [https://www.arca.fiocruz.br/handle/icict/34796].
30. Consoli RAGB, Oliveira RL. Principais mosquitos de importância sanitária no Brasil. Editora Fiocruz. Rio de Janeiro; 1994. 228 p. [https://doi.org/10.7476/9788575412909].
31. Costa-da-Silva AL, Navarrete FR, Salvador FS, Karina-Costa M, Ioshino RS, Azevedo DS et al. Glytube: A Conical Tube and Parafilm M-Based Method as a Simplified Device to Artificially Blood-Feed the Dengue Vector Mosquito, Aedes aegypti. PLoS One. 2013; 8: e53816. [https://doi.org/10.1371/journal.pone.0053816].
32. World Health Organization. Guidelines for laboratory and field testing of mosquito larvicides. World Heal Organ. 2005; 1–41. [https://www.who.int/publications/i/item/WHO-CDS-WHOPES-GCDPP-2005.13].
33. Vidal J, Carbajal A, Sisniegas M, Bobadilla M. Efecto tóxico de Argemone subfusiformis Ownb. y Tagetes patula Link sobre larvas del IV estadio y pupas de Aedes aegypti L. Rev Peru Biol. 2008; 15: 103–9. [https://doi.org/10.15381/rpb.v15i2.1733].
34. Macêdo ME, Consoli RAGB, Grandi TSM, Anjos AMG, Oliveira AB, Mendes NM et al. Screening of Asteraceae (Compositae) Plant Extracts for Larvicidal Activity against Aedes fluviatilis (Diptera: Culicidae). Mem Inst Oswaldo Cruz. 1997; 92: 565–70. [https://doi.org/10.1590/S0074-02761997000400024].
35. Matos JFA. Introdução à Fitoquímica Experimental. 3ª ed. Fortaleza: Editora UFC; 2009. 150 p.
36. Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K. Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res. 2008; 102: 867–73. [https://doi.org/10.1007/s00436-007-0839-6].
37. Musau J, Mbaria J, Nguta J, Mathiu M, Kiama S. Phytochemical composition and larvicidal properties of plants used for mosquito control in Kwale County, Kenya. Int J Mosq Res. 2016; 3: 12–7. [http://erepository.uonbi.ac.ke/handle/11295/100694].
38. Pandey A, Tripathi S. Concept of standardization, extraction and pre phytochemical screening strategies for herbal drug. J Pharmacogn Phytochem. 2014; 2(5): 115–9. E-ISSN: 2278-4136. [https://www.phytojournal.com/archives/2014.v2.i5.255/concept-of-standardization-extraction-and-pre-phytochemical-screening-strategies-for-herbal-drug].
39. Violante IMP, Carollo CA, Silva LI, Oliveira AQC, Pardinho FC, Garcez WS et al. Cytotoxicity and antibacterial activity of scutellarein and carajurone-enriched fraction obtained from the hydroethanolic extract of the leaves of Fridericia chica (Bonpl.) L.G. Lohmann. Nat Prod Res. 2020; 1-7.[https://doi.org/10.1080/14786419.2020.1753050]
40. Lima JCS, Oliveira RG, Silva VC, Sousa PT, Violante IMP, Macho A et al. Anti-inflammatory activity of 4′,6,7-trihydroxy-5-methoxyflavone from Fridericia chica (Bonpl.) L.G.Lohmann. Nat Prod Res. 2020; 34: 726–30. [https://doi.org/10.1080/14786419.2018.1495636].
41. Generoso LA, Araujo PF, Cecílio AB. Avaliação do efeito do óleo essencial da Fridericia chica em diferentes fases de vida do mosquito Aedes aegypti. Rev Sinapse Múltipla. 2020; 9: 47–60. [https://periodicos.pucminas.br/index.php/sinapsemultipla/article/view/21107].
42. Silva HHG, Silva IG, Santos RMG, Rodrigues Filho E, Elias CN. Larvicidal activity of tannins isolated of Magonia pubescens St. Hil. (Sapindaceae) against Aedes aegypti (Diptera, Culicidae). Rev Soc Bras Med Trop. 2004; 37: 396–9. [https://doi.org/10.1590/s0037-86822004000500005] [https://pubmed.ncbi.nlm.nih.gov/15361956/].
43. Raihan SMA. Effect of Plant Flavonoids on Mosquito Larvae. Natl Univ J Sci. 2014; 1(2): 27–30. [chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.nu.ac.bd/uploads/2017/09/03.-EFFECT-OF-PLANT-FLAVONOIDS-ON-MOSQUITO-LARVAE.pdf].
44. Elumalai D, Hemavathi M, Hemalatha P, Deepaa CV, Kaleena PK. Larvicidal activity of catechin isolated from Leucas aspera against Aedes aegypti, Anopheles stephensi, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res. 2016; 115: 1203–12. [https://doi.org/10.1007/s00436-015-4856-6].