ARTIGO DE PESQUISA
Establishment of anthocyanin-producing habituated callus cultures of Tarenaya rosea (Vahl ex DC.) Soares Neto & Roalson
Abstract
A growth regulators-free anthocyanin-producing habituated callus line of Tarenaya rosea was establishment through the selective subculture of cell aggregates from a 2,4-D-dependent anthocyanin callus line to a growth regulators-free MS medium (MS0). After the establishment of the habituated line, new culture conditions were evaluated in order to increase de pigment production. Calluses were transferred to MS0 containing different sucrose concentration (30; 50; 70; 90 g.L-1), total nitrogen concentration (50; 60; 70 mM), NH4+ to NO3- ratio (1:1; 1:2; 1:4; 1:6), and total mineral salt concentration (MS; MS1/2; MS1/4). The most suitable culture conditions to pigment induction have combined to create two new culture formulations, named M1 (70 mM total nitrogen + 70 g.L-1 sucrose) and M2 (1:4 NH4+:NO3- ratio + 70 g.L-1 sucrose). Calluses cultivated on M1 and M2 reached an increase on anthocyanin productivity of 3- and 4-fold, respectively, when compared to cultures maintained in standard MS0 medium. The present work demonstrated the feasibility of eliminating the supplementation with 2,4-D in callus cultures of T. rosea without the loss of anthocyanin production. Moreover, the manipulation of basal medium and sucrose concentration contributed with the increment in anthocyanin content.
- Keywords:
- Cleome rosea.
- Habituation.
- MS medium.
- NH4+:NO3- ratio.
- Total nitrogen.
- Sucrose.
Introduction
Anthocyanins are the most abundant flavonoids widely distributed in plants. These plant pigments are used as natural colorants and additives in foods and beverages, in addition to have nutraceutical and pharmacological potentials[1]. Many studies suggest that anthocyanins play an important role in the prevention of several types of human cancer[2], which demonstrates the importance of anthocyanin consumption in the human diet.
The development of in vitro protocols for anthocyanin production has great interest, since these strategies have been considered to provide a continuous supply of secondary metabolites. The in vitro production circumvents some problems that remain in the extraction of these compounds from natural populations, such as geographical and seasonal variations, as well as the presence of microbial contaminants[3]. However, the in vitro production of plant pigments frequently demands the culture media supplementation with growth regulators[4-6]. Although required, the use of these additives leads to an increase in the production costs and may be a negative factor when considering the application of in vitro systems for commercial production, since many of these substances are not suitable for human consumption. Therefore, the establishment of in vitro cell lines presenting high pigment productivity on a growth regulators-free culture medium has strong commercial significance.
One possibility to reduce or even eliminate the supplementation with growth regulators is the occurrence of habituation, defined as a stable heritable loss in the requirement of growth factors by cultured plant cells in order to induce a given response[7]. Gautheret[8] was the first author to report the phenomenon of habituation under in vitro cultures of carrot. Considering the anthocyanins, many studies related to the production of these pigments by cultured cells indicated the requirement of one or more exogenously-applied growth regulators[9]. On the other hand, some studies reported the development of habituated cultured cells, including anthocyanin-producing cell lines[10-13].
Tarenayarosea, formely named Cleomerosea[14], is a Brazilian native species with medicinal potential[15-17]. Efficient protocols for anthocyanin production were established to the species in the presence of 2,4-dichlorophenoxyacetic acid (2,4-D) for both callus[18] and cell suspension cultures[19]. However, these protocols showed that the pigment production and biomass accumulation required the continuous supplementation of the culture medium with 2,4-D.
The present study reported the establishment of a growth regulators-free anthocyanin-producing callus line of T. rosea and evaluated the pigment production and biomass accumulation in response to modifications in the MS basal medium formulation and sucrose concentration.
Material and Methods
Establishment of callus cultures on growth regulators-free medium
Anthocyanin-producing callus cultures of Tarenaya rosea (Vahl ex DC.) Soares Neto & Roalson were previously established from stem explants cultivated on solidified (7.0 g.L-1 agar) standard MS medium[20], added with 30 g.L-1 sucrose and supplemented with 0.9 µM of 2,4-D[18]. These stock calluses were used in the present work. Four grams of calluses were transferred to flasks (8.0 x 7.0 cm) containing 30 mL of solidified growth regulators-free standard MS medium (MS0), added with 30 g.L-1 sucrose. The flasks were maintained at 24±2ºC under 16 h photoperiod provided by cool white fluorescent tubes (80 µmol.m-2.s-1). Four grams of pigmented cell aggregates were isolated mechanically and subcultivated on fresh MS0 at 20 days interval. This procedure was repeated during 36 cycles (two years) and only callus regions that continued to have pigmented cells and that demonstrated proliferation capacity were selected and subcultivated, until the establishment of an anthocyanin-producing habituated callus line.
Modulation of anthocyanin production
Anthocyanin productivity was evaluated in response to modifications on the MS basal medium formulation and sucrose concentration. Four grams of anthocyanin-producing habituated calluses were transferred to modified MS0 medium containing different sucrose concentration (30; 50; 70; 90 g.L-1), total nitrogen concentration (50; 60; 70 mM), NH4+ to NO3- ratio (1:1; 1:2; 1:4; 1:6) and total mineral salt concentration by diluting MS medium (MS1/2; MS1/4). The cultures were maintained under these conditions for 20 days. At the end of the culture period, the color value index (CV) was quantified (see Anthocyanin extraction and quantification section) and callus biomass accumulation was determined based on fresh (FW) and dry (DW) weight. Dry weight was obtained after drying at 45ºC to constant weight.
Anthocyanin extraction and quantification
Anthocyanin quantification was performed based on the color value index (CV) as reported in Simões et al.[18]. Briefly, samples of calluses (100 mg) were extracted for 24 h at 4ºC using 1% (v/v) HCl-methanol. The extracts were centrifuged (1000 x g for 10 min) and the absorbance was measured at 525 nm with a UV-Vis spectrophotometer (Shimadzu UV - 160). The CV index was calculated using the following equation: CV = 0.1 x OD 525 x 40 /1g FW, where 0.1 x OD 525 is 10% of the absorbance at 525 nm, 40 is the level of dilution (100 mg of callus extracted in 4 mL of HCl-methanol) and FW is the fresh cell weight.
Statistical treatment
Fifteen flasks containing four grams of calluses were cultured per treatment and each experiment was repeated three times. Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA, USA). Measurement data are expressed as the mean ± standard deviation. Means were compared using one‑way Analysis of Variance (ANOVA) or the t-test. For one‑way ANOVA, post hoc testing was performed using Tukey test. P<0.05 was considered to indicate a statistically significant difference.
Results and Discussion
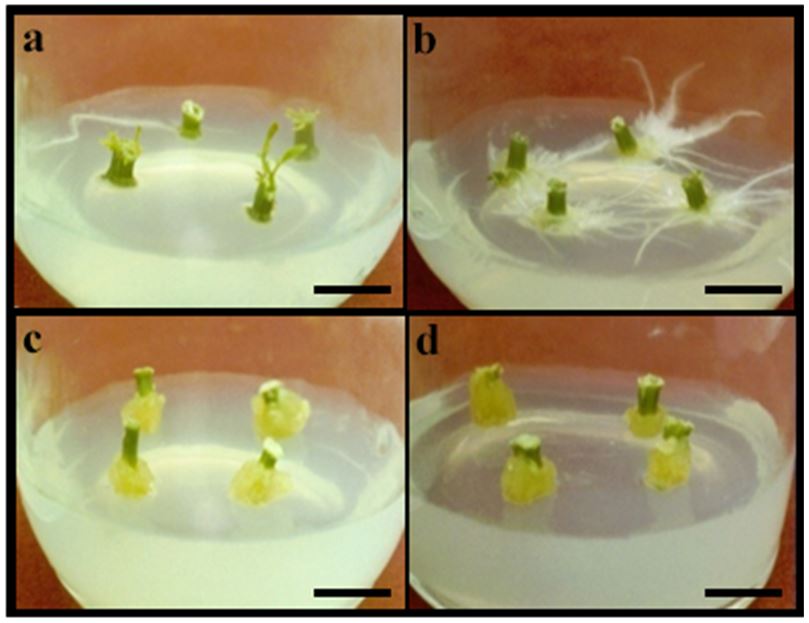
Previous studies[18] demonstrated that stem explants of T. rosea cultivated on MS medium supplemented with different auxins resulted in distinct morphogenic responses, such as the development of aerial parts, rhizogenesis and calogenesis (FIGURE 1a, b, c and d). However, explants inoculated on MS0 remained unresponsive (data not shown). These studies showed that cultures maintained on medium with 2,4-D developed anthocyanin-producing friable calluses with high biomass accumulation, while the transference of calluses to 2,4-D-free medium resulted in a significant reduction of both pigment production and biomass accumulation[18].
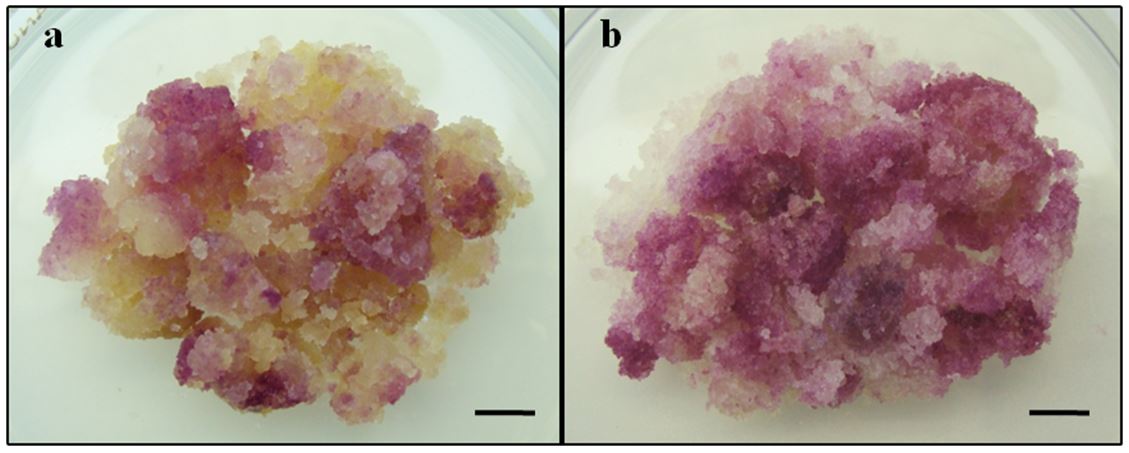
In order to induce a continuous anthocyanin-producing callus line with genetic capacity to pigmented production on MS0, pigmented cell aggregates established in the presence of 2,4-D, were isolated and transferred to MS0. During the cultivation period, the calluses were monitored and only those with pigment and proliferation capacity were selected to be used in the next subculture. After two years under culture (36 subcultures), an anthocyanin-producing habituated callus line was established (FIGURE 2a). In addition, the biomass accumulation in this 2,4-D free-callus line increased 3-fold (12.57±2.15 grams/callus), when compared to the biomass used to initiate each subculture (4.05±0.54 grams/callus). These results are relevant, considering that, as previously mentioned, stem explants of T. rosea inoculated directly on MS0 did not show any morphogenic response. Moreover, it also suggests the presence of habituated cells maintaining the capacity to anthocyanin induction, even without the presence of 2,4-D. The habituation is characterized by exogenous plant hormones autonomy. Although the identification of the mechanism is not completely elucidated, a transcriptome-based analysis of habituated and non-habituated Arabidopsis thaliana plant cell cultures revealed the differential expression of more than 800 genes[21]. Changes at the level of gene expression was also indicated in a comparative biochemical study between a cytokinin habituated and a cytokinin non-habituated callus lines of Glycine max that demonstrated differences in RNA levels and protein profiles[22]. In addition, the epigenetic nature of habituation is shown by the observation that plants regenerated from habituated cell cultures have the original requirement for hormones when cell cultures are established from these plants[23].
Studies reporting the establishment of anthocyanin-producing cell lines that do not require the supplementation with growth regulators are still rare. Habituation of callus cultures for anthocyanin production was obtained in Fragaria ananassa Duch.[12] and in cherry, peach, and Asiatic dayflower[13].
Although the success achieved in establishing a habituated anthocyanin-producing callus line of T. rosea, a potential commercial use required the optimization of pigment production associated with biomass accumulation. Therefore, new assays were performed aiming to optimize the anthocyanin production. Habituated calluses were transferred to modified MS basal medium, as well as to standard MS added with different concentrations of sucrose. Calluses cultured on media supplemented with high sucrose concentrations displayed a significant increase in anthocyanin production (TABLE 1). The highest anthocyanin content, without reduction on biomass accumulation, was achieved in response to 70 g.L-1 sucrose, showing a 2-fold increase on CV index. Sugars are required as substrates for anthocyanins biosynthesis and many studies reported a strong influence of sucrose concentration on in vitro induction of these pigments[24-26]. However, cultures of T. rosea maintained in the presence of 90 g.L-1 sucrose showed a 50% reduction in biomass accumulation. The decrease in cell proliferation, probably related to inhibition of nutrient uptake due to the increased in medium osmotic potential, was also observed in the 2,4-D-dependent anthocyanin callus line of T. rosea[18].
| MS medium | CV/gFW | FW (g) | DW (g) |
|---|---|---|---|
| Sucrose (g L-1) | |||
| 30 | 4.22±1.26b | 12.57±2.15a | 0.38±0.04c |
| 50 | 7.94±0.96ab | 11.47±0.67a | 0.54±0.06b |
| 70 | 10.25±1.22a | 11.01±1.98a | 0.80±0.12a |
| 90 | 12.75±4.31a | 6.74±0.98b | 0.55±0.06b |
| Total nitrogen (mM) | |||
| 50 | 6.09±2.75b | 12.91±0.68a | 0.42±0.02a |
| 60 | 4.22±1.26b | 12.57±2.15a | 0.38±0.04a |
| 70 | 11.02±1.59a | 13.46±1.07a | 0.44±0.09a |
| NH4+:NO3- | |||
| 1:1 | 3.48±1.26b | 9.09±0.41b | 0.26±0.01b |
| 1:2 | 4.22±1.26b | 12.57±2.15ab | 0.38±0.04ab |
| 1:4 | 11.85±4.98a | 13.38±1.38a | 0.35±0.04ab |
| 1:6 | 7.22±0.59ab | 13.13±1.94ab | 0.41±0.10a |
| Mineral salt | |||
| MS | 4.22±1.26b | 12.57±2.15a | 0.38±0.04a |
| MS1/2 | 10.43±0.84a | 8.46±0.79b | 0.31±0.02ab |
| MS1/4 | 7.23±2.42ab | 6.39±1.06b | 0.19±0.04c |
| Data represent mean ± standard deviation. Same letters in columns of each treatment are not significantly different by Tukey test at 5%. CV - Color value index; FW - Fresh weight; DW - Dry weight The standard MS medium conditions are indicated in bold. |
|||
The total nitrogen concentration in the MS medium also influenced the pigment production. The nitrogen has important functions in the development of plants such as to act as signaling molecule regulating the expression of a variety of genes[27]. The higher anthocyanin content was reached by calluses maintained on medium formulated with an increase in total nitrogen (70 mM) when compared to the standard concentration of MS medium (60 mM) (TABLE 1). Calluses cultivated on medium with 70 mM increased more than 2-fold their pigment production, without influence on biomass accumulation. Similar results were observed in cultures of carrot cells[28]. On the other hand, changes in the total nitrogen concentration of MS medium, caused a low anthocyanin production in 2,4-D-dependent callus line of T. rosea[18].
The NH4+:NO3- ratio also influenced the anthocyanin productivity in habituated calluses of T. rosea. On MS medium formulated with 1:4 ratio of NH4+ to NO3-, pigment content increased more than 2-fold when compared to the standard MS medium (1:2 NH4+:NO3- ratio), without influence on biomass accumulation (TABLE 1). The manipulation of NH4+ to NO3- ratio also markedly affected in vitro production of anthocyanin pigments in callus cultures of T. rosea in the presence of 2,4-D[18], as well as in other species[26,28].
The whole concentration of macro- and micronutrients in culture medium was also evaluated through the dilution of the total mineral salt concentration of MS (MS1/2 and MS1/4). Calluses cultivated on diluted MS medium resulted in high values of CV index, but with a significant reduction on biomass accumulation (TABLE 1). Although the nutritional restriction is a strategy used to induce secondary metabolites under in vitro conditions, it is frequently associated to a decrease in biomass accumulation[29].
Based on the results described above, two new culture medium formulations were established associating the best culture conditions for anthocyanin production. They were named M1 (MS + 70 mM total nitrogen + 70 g.L-1 sucrose) and M2 (MS + 1:4 ratio of NH4+ to NO3- + 70 g.L-1 sucrose). Calluses transferred to these new formulations maintained a high biomass accumulation and reached higher values of CV indexes (TABLE 2). The CV index achieved by calluses transferred to M1 (15.47±2.16) represented an increment in anthocyanin production of more than 3-fold when compared to cultures maintained on standard MS0 + 30 g.L-1 sucrose (4.22±1.26). However, the highest anthocyanin production was observed on M2 (FIGURE 2b), reaching more than 4-fold increase in the CV index (19.72±1.11). This anthocyanin content was even higher than the productivity obtained by the 2,4-D-dependent callus line of T. rosea[18].
| MS medium | CV/gFW | FW (g) | DW (g) |
|---|---|---|---|
| M1 | 15.47±2.16b | 12.76±1.87a | 0.74±0.14a |
| M2 | 19.72±1.11a | 12.09±2.37a | 0.84±0.09a |
| Data represent mean ± standard deviation Same letters in each column are not significantly different by t-test at 5%. CV - Color value index; FW - Fresh weight; DW - Dry weight M1 = modified MS (70 mM total nitrogen) + 70 g.L-1 sucrose M2 = modified MS (1:4 ratio of NH4+ to NO3-) + 70.g L-1 sucrose |
|||
Conclusion
These results demonstrated the efficiency in the continuous production of anthocyanin in calluses of T. rosea maintained in the absence of growth regulators supplementation, showing that the culture became habituated to the pigment induction. In addition, the manipulation of the basal medium and sucrose concentration proved to be an efficient strategy to improve the anthocyanin productivity, representing a good perspective of potential commercial use of these in vitro cultures.
Financing Source
This work was supported by the Brazilian Council for Scientific and Technological Development (CNPq) and The Carlos Chagas Filho Research Support Foundation (FAPERJ).
Conflict of Interests
There is no conflict of interests.
Acknowledgments
The authors are grateful to Ivan Gonçalves Ribeiro (Qualitec/UERJ), Aline Medeiros Saavedra de Paula (Proatec/UERJ) and Adriana Maria Lanziotti (TCT/FAPERJ) for the valuable technical assistance.
Contributors
Study design: CS-G; NA
Data curation: CS-G; LSC
Data collect: CS-G; LSC
Data analysis: CS-G; LSC; TCC; NA
Writing of the original manuscript: CS-G; LSC; TCC; NA
Proofreading and Editing: CS-G; LSC; TCC; NA.
References
1. Khoo HE, Azlan A, Tang ST, Lim SM. Anthocyanidins and anthocyanins: colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr Res. 2017; 61: 01-21. [https://doi.org/10.1080/16546628.2017.1361779].
2. Lin B-W, Gong C-C, Song H-F, Cui Y-Y. Effects of anthocyanins on the prevention and treatment of cancer. Br J Pharmacol. 2017; 174: 1226-1243. [https://doi.org/10.1111/bph.13627].
3. Davis KM, Deroles SC. Prospects for the use of plant cell cultures in food biotechnology. Curr Opin Biotechnol. 2014; 26: 133-140. [https://doi.org/10.1016/j.copbio.2013.12.010].
4. Arghavani P, Haghbeen K, Mousavi A. Enhancement of Shikalkin Production in Arnebia euchroma Callus by a Fungal Elicitor, Rhizoctonia solani.Iran J Biotech. 2015; 13: 10-16. [https://doi.org/10.15171/ijb.1058].
5. Lage DA, Tirado MS, Vanicore SR, Sabino KCC, Albarello N. Production of betalains from callus and cell suspension cultures of Pereskia aculeata Miller, an unconventional leafy vegetable. Plant Cell Tiss Organ Cult. 2015; 122:341-350. [https://doi.org/10.1007/s11240-015-0771-x].
6. Rocha AS, Rocha EK, Alves LM, Moraes BA, Castro TC, Albarello N, Simões-Gurgel C. Production and optimization through elicitation of carotenoid pigments in the in vitro cultures of Cleome rosea Vahl (Cleomaceae). J Plant Biochem Biotechnol. 2015; 24: 105-113. [https://doi.org/10.1007/s13562-013-0241-7].
7. Meins F. Habituation: heritable variation in the requirement of cultured plant cells for hormones. Annu Rev Genet. 1989; 23: 395-408. [https://doi.org/10.1146/annurev.ge.23.120189.002143].
8. Gautheret RJ. Hetero-auxines et cultures de tissus végétaux. Bull Soc Chim Biol. 1942. 24: 13-46.
9. Simões C, Albarello N, Castro TC, Mansur E. Production of anthocyanins by plant cell and tissue culture strategies. In: Erdogan-Orhan I. (Org.). Biotechnological production of plant secondary metabolites. Bentham Science Publishers, 2012. p. 67-86.
10. Park K, Saimoto H, Nakagawa S, Sakurai A, Yokota T, Takahashi N et al. Occurrence of brassinolide and castasterone in crown gall cells of Catharanthus roseus. Agric Biol Chem. 1989; 53: 805-811. [https://doi.org/10.1080/00021369.1989.10869357].
11. Kevers C, Filali M, Petit-Paly G, Hagege D, Rideau M, Gasper TH. Habituation of plant cells does not mean insensitivity to plant growth regulators. In Vitro Cell Dev Biol. 1996; 32: 204-209. [https://doi.org/10.1007/BF02822767].
12. Asano S, Ohtsubo S, Nakajima M, Kusunoki M, Kaneko K, Katayama H et al. Production of anthocyanins by habituated cultured cells of Nyoho strawberry (Fragaria ananassa Duch.). Food Sci Technol Res.2002; 8: 64-69. [https://doi.org/10.3136/fstr.8.64].
13. Asano S, Otobe K. Production of phytochemicals by using habituated and long-term cultured cells. Plant Biotechnol. 2011; 28: 51-62. [https://doi.org/10.5511/plantbiotechnology.10.1109a].
14. Soares Neto RL, Thomas WW, Barbosa MRV, Roalson EH. New combinations and taxonomic notes for Tarenaya (Cleomaceae). Acta Bot Bras. 2018; 32: 540-545. [https://doi.org/10.1590/0102-33062017abb0417].
15. Simões-Gurgel C, Mattos JCP, Sabino KCC, Caldeira-de-Araújo A, Coelho MGP, Albarello N et al. Medicinal potential from in vivo and acclimatized plants of Cleome rosea Vahl ex DC. (Capparaceae). Fitoterapia. 2006; 77: 94-99.
16. Simões-Gurgel C, Castro TC, Cordeiro LS, Albarello N, Mansur E, Romanos MTV. Antiviral activity of Cleome rosea extracts from field-grown plants and tissue culture-derived materials against acyclovir-resistant Herpes simplex viruses type 1 (ACVr-HSV-1) and type 2 (ACVr-HSV-2). World J Microb Biot. 2010; 26: 93-99. [https://doi.org/10.1007/s11274-009-0173-5].
17. Simões-Gurgel C, Rocha AS, Cordeiro LS, Gayer CRM, Castro TC, Coelho MGP et al. Antibacterial activity of field-grown plants, in vitro propagated plants, callus and cell suspension cultures of Cleome rosea Vahl. J Pharm Res. 2012; 5: 3304-3308.
18. Simões-Gurgel C, Bizarri CHB, Cordeiro LS, Castro TC, Coutada LCM, Silva AJR et al. Anthocyanin production in callus cultures of Cleome rosea: modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiol Biochem.2009; 47: 895-903. [https://doi.org/10.1016/j.plaphy.2009.06.005].
19. Simões-Gurgel C, Cordeiro LS, Castro TC, Callado CH, Albarello N, Mansur E. Establishment of anthocyanin-producing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tiss Org Cult. 2011; 106: 537-545. [https://doi.org/10.1007/s11240-011-9945-3].
20. Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962; 15: 473-497. [https://doi.org/10.1111/j.1399-3054.1962.tb08052.x].
21. Pischke MS, Huttlin EL, Hegeman AD, Sussman MR. A transcriptome-based characterization of habituation in plant tissue culture. Plant Physiol. 2006; 140: 1255-1278. [https://doi.org/10.1104/pp.105.076059].
22. Plessis S, Stirk WA, Cress WA, van Stade J. Biochemical comparisons of habituated and non-habituated callus lines of Glycine max (L.) cv. Acme. Plant Growth Regul. 1996; 18: 223-231. [https://doi.org/10.1007/BF00024386].
23. Smulders MJM, de Klerk GJ. Epigenetics in plant tissue culture. Plant Growth Regul. 2011; 63: 137-146. [https://doi.org/10.1007/s10725-010-9531-4].
24. Pasqua G, Monacelli B, Mulinacci N, Rinaldi S, Giaccherini C, Innocenti M et al. The effect of growth regulators and sucrose on anthocyanin production in Camptotheca acuminata cell culture. Plant Physiol Biochem. 2005; 43: 293-298. [https://doi.org/10.1016/j.plaphy.2005.02.009].
25. Solfanelli C, Poggi A, Loreti E, Alpi A, Perata P. Sucrose specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006; 140: 637-646. [https://doi.org/10.1104/pp.105.072579].
26. Ram M, Prasad KV, Kaur C, Singh SK, Arora A, Kumar S. Induction of anthocyanin pigments in callus cultures of Rosa hybrida L. in response to sucrose and ammonical nitrogen levels. Plant Cell Tiss Organ Cult. 2011; 104: 171-179. [https://doi.org/10.1007/s11240-010-9814-5].
27. Peng M, Hudson D, Schofield A, Tsao R, Yang R, Gu H et al. Adaptation of Arabidopsis to nitrogen limitation involves induction of anthocyanin synthesis, which is controlled by the NLA gene. J Exp Bot. 2008; 59: 2933-2944. [https://doi.org/10.1093/jxb/ern148].
28. Narayan MS, Venkataraman LV. Effect of sugar and nitrogen on the production of anthocyanin in cultured carrot (Daucus carota) cells. J Food Sci. 2002; 67: 84-86. [https://doi.org/10.1111/j.1365-2621.2002.tb11363.x].
29. Schiozer AL, Barata LES. Stability of natural pigments and dyes. Rev Fitos. 2007; 3: 6-23. [https://doi.org/10.32712/2446-4775.2007.71].