QUÍMICA
Chemical composition, ethnopharmacology and biological activity of Geissospermum Allemão species (Apocynaceae Juss.)
Composição química, etnofarmacologia e atividade biológica de espécies de Geissospermum Allemão (Apocynaceae Juss.)
Abstract
The chemistry and pharmacology literature on Geissospermum is surveyed. Pau-pereira or pao pereira (G. laeve (Vell.) Miers; G. vellosii Fr. All. is a synonym) is the best known of approximately six Geissospermum species. Pau-pereira bark is mainly used for its bitterness and medicinal properties. It is used to treat pain, liver ailments, fevers, appetite loss, indigestion, dizziness, constipation and malaria, among other ailments. The other Geissospermum species have similar traditional uses. Pau-pereira provides monoterpenoid indole alkaloid rich extracts and fractions and pure isolates that comprise formulations used in clinical practice for the treatment of prostate cancer (flavopereirine) and HIV-AIDS (flavopereirine). Also, G. vellosii extracts exhibit pronounced antinociceptive activity in animals (perhaps due to 12-methoxy-1-methylaspidospermine). Induced amnesia in mice is reduced by pau-pereira extracts and geissospermine which also have anticholinesterase activity and potential application against Alzheimer's Disease. G. argenteum Woodson extracts exhibit in vitro antibacterial activity. Extracts of three Geissospermum species and isolated alkaloids aspidocarpine, flavopereirine, and geissolosimine strongly inhibit the human malaria parasite Plasmodium falciparum Welch in vitro. Two Geissospermum species inhibit Plasmodium yoelii in rodents and exhibit toxicity. Geissospermum extracts and alkaloids inhibit P. falciparum, Leishmania infantum Nicolle and Trypanosoma cruzi Chagas and deserve further study as therapeutic agents.
- Keywords:
- pau-pereira.
- anticancer.
- anti-HIV.
- anti-Leishmania.
- anti-Trypanosoma.
- antimalarial.
Resumo
A literatura de química e farmacologia sobre Geissospermum é revisada. Pau-pereira ou pao pereira (G. laeve (Vell.) Miers; G. vellosii Fr. All. é sinonímio) é a mais conhecida de aproximadamente seis espécies de Geissospermum. A casca de pau-pereira é usada principalmente por sua amargura e propriedades medicinais. É usada para tratar dor, doenças de fígado, febres, perda de apetite, mal-digestão, tontura, prisão de ventre e malária, entre outras enfermidades. A outra espécie de Geissospermum tem usos tradicionais semelhantes. Pau-pereira é fonte de alcaloides indólicos monoterpenoídicos isolados e extratos e frações enriquecidos nesses alcaloides que são utilizados na clínica médica para o tratamento do câncer de próstata (flavopereirina) e HIV-AIDS (flavopereirina). Também, os extratos de G. vellosii exibem atividade antinociceptíva em animais (talvez devido a 12-metoxi-1-metilaspidospermina). Amnesia induzida em camundongos é reduzida por extratos de pau-pereira e geissospermina que também possuem atividade anticolinesterase e aplicação potencial contra o mal de Alzheimer. Extratos de G. argenteum Woodson exibem atividade antibacteriana. Extratos de três espécies de Geissospermum e os alcaloides isolados aspidocarpina, flavopereirina e geissolosimina inibem fortemente o parasito da malária humana Plasmodium falciparum Welch in vitro. Duas espécies de Geissospermum inibem Plasmodium yoelii em roedores e exibem toxicidade. Os extratos de Geissospermum e alcaloides inibem P. falciparum, Leishmania infantum Nicolle e Trypanosoma cruzi Chagas e merecem estudos futuros como agentes terapêuticos.
- Palavras-chave:
- pau-pereira.
- anticâncer.
- anti-HIV.
- anti-Leishmania.
- anti-Trypanosoma.
- antimalárico.
Botanic aspects of Geissospermum Allemão
According to (Ribeiro et al., 1999), this genus is comprised of trees with alternate leaves, furrowed trunk and little or no latex. Lorenzi (2002) reports that this genus produces latex in the fruit and the extremities of the branches. The trees of this genus exhibit heavy, yellowed sapwood, differentiated from the darker heartwood; regular and irregular grain, fine texture, indistinct smell and bitter taste. The Plant List (TPL, 2014) recognizes six Geissospermum species all of which are native to Brazil (TABLE 1). They occur in the terra firme woods of the Amazon region, mainly in Amazonas and Pará States and also in the northeast, southeast and south of Brazil (Forzza, 2010) and in other countries of the Amazon region (WCSP, 2014). Importantly, G. laeve is the accepted name and G. vellosii is a synonym however the species cited in each work surveyed was maintained throughout the text.
| Accepted Name1 | Synonym1 | Distribution2,3 |
| G. argenteum Woodson | N. South America: N. Brazil (AM, AP, RO) to Venezuela, Guianas | |
| G. fuscum Markgr. | S. Venezuela to N. Brazil | |
| G. laeve (Vell.) Miers | G. martianum Miers G. vellosii Fr. All. Tabernaemontana laevis Vell. | N. South America: Guianas to Brazil (AP, PA, AM, MA, BA, DF, MG, ES, RJ) |
| G. reticulatum A. H. Gentry | S. Venezuela, Peru, N. Brazil (AC, RO) to N. Bolivia | |
| G. sericeum Benth. & Hook. f. | N. South America: Guianas, Brazil (AP, AM, AC, RO, MA) | |
| G. urceolatum A. H. Gentry | N. Brazil (PA, AM) | |
| 1TPL (2014); 2WCSP (2014); 3Forzza (2010). | ||
Chemistry of Geissospermum species
Studies on the chemical composition of Geissospermum species began at the end of the 19th century in Brazil and Europe. G. vellosii (synonym for G. laeve), known as pau-pereira, was the first species of this genus to undergo studies on chemical composition. From this species, many alkaloids were isolated. Fewer chemical studies have been performed on the other 5 species of Geissospermum.
Alkaloids of G. laeve
The most important alkaloid isolated from G. laeve is geissospermine (1) (FIGURE 1). Isolated for the first time by O. Hesse in 1877 (Puiseux et al., 1959), 1 (isolated from G. vellosii) was subjected to acid hydrolysis and yielded cleavage products geissoschizine (2), apogeissoschizine (3) and geissoschizoline (4) (Rapoport et al., 1958). The structure of 2 was proposed by (Rapoport et al., 1958) and confirmed by (Puiseux et al., 1959). The structures of the cleavage products 2 and 3 together with a partial structure of 1 were later proposed (Rapoport et al., 1960). The stereochemistry of 1 was established by X-ray crystallography and by NMR methods (Chiaroni et al., 1976; Goutarel et al., 1978). Chiaroni and Riche (1979) isolated 1 from G. laeve and re-confirmed that acid hydrolysis of 1 provided 2 and 4. Recently, alkaloids 1, 2 and 4 were found to be the main components of G. vellosii bark, with other classes of compounds (Werner et al., 2009).
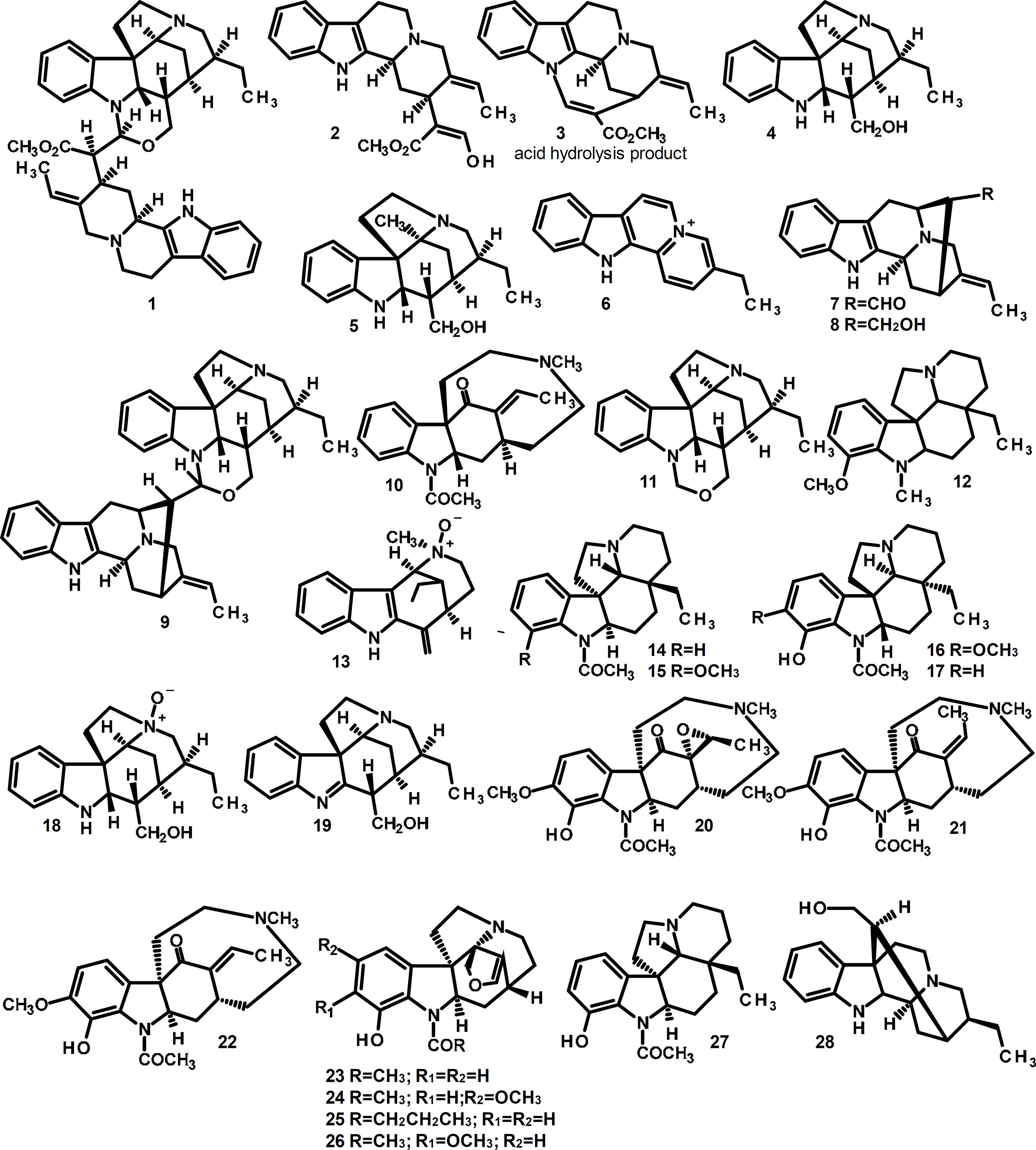
Pereirine (5) (FIGURE 1) was the first alkaloid isolated in Brazil. It was obtained as a mixture from pau-pereira by the pharmacist Ezequiel Corrêa dos Santos. However, this isolation of 5 was not recognized in Europe (Almeida et al., 2009). Bertho and Koll (1961) determined the structure of 5 isolated from pau-pereira.
(Bejar et al., 1957) and Hughes and Rapoport (1958) independently isolated the strong base flavopereirine (6) (cited species were G. laeve and G. vellosii, respectively) and elucidated its structure (FIGURE 1). Ban and Seo (1961) reported methods for the synthesis of 6 and other β-carboline derivatives. Later, Wenkert and Kilzer (1962) synthesized 6 using then novel methods in indole alkaloid synthesis such as mercuric acetate oxidation and palladium-induced conversion.
Rapoport and Moore (1962) isolated three monoterpenoid indole alkaloids from G. vellosii: vellosimine (7), vellosiminol (8) and geissolosimine (9) (FIGURE 1) through fractionation of the alkaloid-rich pH 7 fraction (Manske and Harrison, 1965). In 1973, geissovelline (10) was isolated from G. vellosii (Moore and Rapoport, 1973). Recently, high performance counter-current chromatography (HPCCC) and electrospray ionization tandem mass spectrometry (ESI-MSn) techniques were applied to the isolation of 1, 4, 8 and 9 and geissoschizone (11) from G. vellosii stem bark crude methanol extract. The electrospray ionization mass spectrometry fragmentation behavior of these alkaloids was also established (Mbeunkui, Grace and Lila, 2012; Mbeunkui et al., 2012). Werner and collaborators (2009) isolated the alkaloid 12-methoxy-1-methylaspidospermidine (12) from the bark of G. vellosii. Pausperadine A (13) was isolated from G. vellosii by Ishiyama and collaborators (2005).
Alkaloids of G. argenteum, G. reticulatum and G. sericeum
Paccioni and Husson (1978) studied G. argenteum bark and leaves collected in French Guiana. Chloroform extraction, basification, followed by column chromatography led to the isolation of (-)-demethoxyaspidospermine (14), (-)-aspidospermine (15), (+)-aspidoscarpine (16) and (+)-demethylaspidospermine (17). Steele and collaborators (2002) isolated 4, 6, geissoschizoline N4-oxide (18) and 1,2-dehydrogeissoschizoline (19) from the water-methanol extracts of G. sericeum bark (from Roraima State, Brazil). Recently, aspidospermatantype monoterpene indole alkaloids 20-26 were isolated from the leaves and bark of G. reticulatum (Reina et al., 2012). The absolute configuration was established for (+)-2R,7R,15R,17S,19S-10-demethoxy-12-hydroxy17,19-epoxygeissovelline (20) by vibrational circular dichroism (VCD). The handedness of geissovelline derivatives 21 and 22, geissospermidine (23) and related geissospermidine derivatives 24-26 was deduced based on their connectivity, relative stereochemistry, and the determination of skeletal chiral centers 2R,7R,15R of 20 (Gordillo-Roman et al., 2013). Also, O-demethylaspidospermine (27), geissosreticulatine (28) and 6 were isolated from G. reticulatum in this work.
Names for Geissospermum species and non-medicinal uses
Geissospermum species are known in different parts of Brazil by the names: pau-pereira, quinarana ("false quinine"), quina-quina (a reference to the bitterness of quinine), acari, acariquara, acariquara branca, among other names. Plants of this genus are known for their extreme bitterness. Acariquara branca is used in civil construction for the manufacture of posts and poles for the support of telephone and power lines, supports for houses in inundated areas and for the production of handles for different tools and agricultural implements (hammers, hoes, spades, shovels, etc.).
Ethnobotanic and other literature on medicinally-useful Geissospermum species
Besides these uses, the bark of Geissospermum species is highly valued for its medicinal properties (Lorenzi, 2002). Pau-pereira (G. vellosii) was considered by Gustavo Peckolt (1861-1923) to be one of the ten most important Brazilian medicinal plants (Santos, Pinto and Alencastro, 1998). The bark of G. vellosii is frequently used to prepare traditional remedies for the treatment of liver problems, fever, malaria, dizziness, stomach disorders, appetite loss, indigestion, constipation, pain and is used as an aphrodisiac (Muñoz et al., 2000; Jácome, Souza and Oliveira, 2003; Werner et al., 2009). Its curative powers are associated with its bitterness. G. vellosii (pau-pereira) was cited in the first edition of the Brazilian Pharmacopeia. Pau-pereira may be suffering from diminished use (availability) and species authenticity in medicinal plant markets in Brazil (Brandão et al., 2013). In their literature survey on Brazilian medicinal plant research, Brito and Brito (1993) reported that G. vellosii extracts are used as: neuromuscular blocker, anticholinesterase agents and hypotensives. The active principle cited for these and other biological activities is flavopereirine (6).
Geissospermum species bark is generally cited in the ethnobotanic literature for the treatment of malaria. Brandão and collaborators (1992) performed a field survey of traditionally used antimalarial plants in locations in south Pará and northeast Rondônia State in the Brazilian Amazon region and found that G. sericeum was used. Furthermore, Oliveira and collaborators (2003) found G. sericeum to be among the most important antimalarial plants in a survey of 108 literature sources on ethnomedical uses of plants. Also, G. sericeum was among the antimalarial plants revealed by an ethnobotanic survey by Milliken (1997) conducted among the indigenous groups of the savanna and forest of Roraima State in the northern Brazilian Amazon region. G. sericeum bark decoctions can cause abortions. Nevertheless, they are used in the treatment of malaria by the Wapixana, Wai-Wai, Macuxi, Taurepang and Xiriana (Yanomami) peoples. G. sericeum was said by one of the informants to be used in association with Citrus aurantufolia roots (Rutaceae) in the prophylaxis of malaria. Also, G. sericeum and other antimalarial plants are used as abortifacients and plant choice is based on the extremely bitter taste which may have some relation to the toxicity.
An ethnopharmacological study in French Guiana revealed the common use of 34 plant species as antimalarials in traditional preparations. Modern drugs were also in regular use among the group studied. Many individuals combine modern drugs and traditional preparations so as to guarantee a real cure. The plant is believed to purify the blood and the liver. Geissospermum species were said also to be used for the prevention (prophylaxis) of malaria (Vigneron et al., 2005).
Ethnopharmacology and biological activity of Geissospermum species
There are a variety of medicinal uses for Geissospermum extracts and isolated alkaloids. This includes clinically-used standardized extracts in anti-cancer formulations and an anti-HIV formulation based on pure isolated substances. Geissospermum extracts and or alkaloids exhibit in vitro and in vivo anticholinesterase and antinociceptive, in vitro antimicrobial, in vitro anti-Trypanosoma and anti -Leishmania, and in vitro and in vivo (in mice) anti-malarial activities. The literature on the pharmacological activity of Geissospermum species extracts and isolated alkaloids is surveyed in the next sections.
Anti-cancer activity and clinical usefulness of pau-pereira (G. laeve)
Bemis and collaborators (2009) tested the in vitro and in vivo effects of a G. vellosii extract against the human prostate cancer LNCaP cell line. This extract strongly inhibited the growth of cultures of LNCaP cells in a dose-dependent manner and induced apoptosis. Extract was administered orally over 6 weeks to immunodeficient mice xenografted with LNCaP cells. Tumor growth was suppressed by up to 80% in some groups compared with tumors in vehicle-treated mice (controls). Lower doses (10 or 20 mg/kg/day), not higher ones, were best at inducing tumor cell apoptosis and in reducing tumor cell proliferation and xenograft growth compared to controls.
A natural formulation was developed comprised of pau-pereira (G. vellosii) bark extract and Rauwolfia vomitoria Afzel extract (Apocynaceae) for the prevention and treatment of prostate cancer. This phytotherapeutic agent contains the β-carboline flavopereirine (6) from pau-pereira and the alkaloid alstonine from Rauwolfia vomitoria in the proportion 3-4:1, respectively. These alkaloids exhibit different mechanisms of action in the prevention and control of cancer. The mechanism of action of this natural formulation is based on the induction of apoptosis (programmed cell death) through the destabilization of the DNA of cancerous cells. At proper formulation doses, the mechanism is selective for neoplasic cells. It is claimed that this natural medicine reduces the levels of PSA (prostate specific antigen), an important metabolic marker for this type of cancer. It is also said to attenuate symptoms such as benign prostatic hyperplasia (BPH). The preparation of this formulation involves partial purification to remove toxic compounds (Hall and Beljanski, 2005).
The Beljanski alternative cancer protocol based on G. vellosii and R. vomitoria is recognized in medical circles and is the subject of ongoing research to establish the extent to which it can be used against different forms of prostate cancer (Block, 2014). Recently the effects of G. vellosii extracts on castration-resistant prostate cancer (CRPC) have been examined in vitro. Pau-pereira (called pao-pereira or pao) extracts induce cell growth arrest and apoptosis, partially through inhibiting NF-κB activation in prostate cancer cells and may protect against CRPC (Chang et al., 2014).
Pau-pereira extracts exhibit in vitro and in vivo cytotoxicity to ovarian cancer cells and also exhibit synergistic effects when combined with the ovarian cancer drug carboplatin. In vivo, pau-pereira extract alone suppressed tumor growth in a mouse model of preclinical ovarian cancer by 79% and decreased ascites by 55%. A pau-pereira-carboplatin combination increased tumor inhibition to 97% and ascites were completely suppressed. These extracts could enhance the effects of carboplatin and have potential in ovarian cancer chemotherapy (Yu and Chen, 2014).
Anti-HIV activity of flavopereirine
A pharmaceutical composition was developed based on flavopereirine (6) for the treatment of HIV (human immunodeficiency virus) infections. The formulation consists of 250-500 mg orally administered or 200-600 βg intradermally injected active principle (Beljanski, 1994; 2005).
Antinociceptive activity of G. laeve
In their survey of sources of Brazilian plants from the 16th to 19th century with possible action on the central nervous system (CNS), Giorgetti, Rossi and Rodrigues (2011) revealed preliminary work demonstrating the inhibition of serotonin capture by alkaloids from G. laeve (Barros et al., 2006). Later, Werner and collaborators (2009) demonstrated that the crude ethanol extract and a chloroform fraction obtained from G. vellosii Fr. All. exhibited pronounced antinociceptive effects in chemical models of nociception. These models involved induced pain produced by acetic acid and formalin in mice. The doses used (1, 10, 30 and 100 mg/kg of animal weight) did not interfere with the locomotion of the animals. The antinociceptive effect of the dichloromethane fraction involved an interaction with the serotoninergic 5-HT1A system. The alkaloid that was isolated and identified as being a participant in this interaction was 12-methoxy-1-methyl-aspidospermine (12) which contributed to the explanation of the antinociceptive properties of the plant extract.
Anticholinesterase activity of G. laeve
Antiacetylcholinesterase activity of an alkaloid-rich fraction from G. vellosii stem bark has been studied (Lima et al., 2009). This fraction inhibited rat brain and electric eel acetylcholinesterases, and horse serum butyrylcholinesterase in a concentration-dependent manner with median inhibition concentration (IC50) values of 39.3, 2.9 and 1.6 mg/mL, respectively. The effect of this alkaloid fraction from G. vellosii trunk bark on the memory of mice was also explored. The main alkaloid in this fraction was geissospermine (1) and it promoted reduction in scopolamine-induced amnesia in mice. This model of cholinergic deficit is validated for the development of drugs used in the symptomatic treatment of Alzheimer's Disease. Thus, 1 exhibits anticholinesterase activity and can reverse cognitive deficits in models based on cholinergic hypofunction.
In follow up work, a docking study was performed on 1 in the active site of acetylcholinesterase (AChE).
This allowed predictions of optimal "bioactive" conformation and binding orientation for 1 that exhibited a large number of interactions between specific functional groups of geissospermine and AChE amino acid residues (Araújo et al., 2011).
Antimicrobial activity of G. argenteum
The antimicrobial activity of crude ethanol extracts of 10 species of plants found in Amapá State in the Brazilian Amazon forest was studied (Correia et al., 2008). G. argenteum, known by the common name quinarana-da-fruta-pequena, was one of the plants collected. The diffusion in agar protocol was used to evaluate antimicrobial activity. The procedure was carried out by impregnating paper discs that are 6 mm in diameter with ethanol extracts and then evaporating the ethanol to obtain quantities of extract of 80 to 1.25 μg on the paper discs. The extracts were tested against ATCC (American Type Culture Collection) and multi-drug-resistant strains obtained from a hospital (clinical) collection. In this study, G. argenteum exhibited activity against multi-drug-resistant Staphylococcus aureus (minimum inhibitory concentration (MIC) of 5 μg). In tests against ATCC strains, these extracts exhibited activity against Pseudomonas aeruginosa (MIC 20 μg) and against S. aureus (MIC 80 pg).
In a more recent study, the antimicrobial activity of extracts and fractions of G. argenteum collected in Roraima State, Brazil was evaluated. The leaf water and bark methanol extracts, as well as the ethyl acetate fraction of the bark methanol extract and the water-methanol fraction of the bark methanol extract were found to be partially active against S. aureus. Bark methanol extract and the hexane fraction of the bark methanol extract were partially active against S. mutans. Extracts and fractions tested were inactive against Escherichia coli and Candida albicans (Camargo, 2011).
Anti-Leishmania and anti Trypanosoma activities of G. reticulatum extracts and alkaloids
G. reticulatum extracts and isolated alkaloids exhibit inhibitory activity against protozoan parasites (Reina et al., 2012). O-demethylaspidospermine (27) was very active against Leishmania infantum (Concentration that inhibits cell growth by 50%, GI50 = 7.7 mg/mL) and less active against Trypanosoma cruzi (GI50 = 41.7 mg/mL). The toxicity of 27 against CHO cells (GI50 = 16.7 mg/mL) was lower than that of the reference drugs amphotericin B and nifurtimox (GI50 = 10.3 and 13.9 mg/mL, respectively). N-Deacetyl-N-butanoylgeissospermidine (25) moderately inhibi ted L. infantum (GI50 = 52.2 mg/mL).
Antimalarial activity of Geissospermum extracts and alkaloids
A survey of traditionally used antimalarial plants from the Brazilian Amazon found that G. sericeum trunk bark extracts were inactive against P. berghei in vivo (Brandão et al., 1992). In an independent study, the bark methanol extract of G. argenteum was considered to be highly active as it exhibited in vitro antiplasmodial activity (IC50 = 4.6 μg/mL) against P. falciparum K1 strain (Camargo, 2011). As discussed above, aspidocarpine (16) is a component of the bark extracts of G. argenteum (Paccioni and Husson, 1978). This compound (isolated from Aspidosperma desmanthum (Henrique, Nunomura and Pohlit, 2010) has been shown to exhibit high in vitro activity (IC50 = 19 nM) against P. falciparum K1 strain (Andrade-Neto et al., 2007).
In a study of antimalarial plants used traditionally by a Chácobo Amerindian community in the Bolivian Amazon, the G. laeve trunk bark ethanol-water extract exhibited good in vitro inhibitory activity against the chloroquine-sensitive F32 strain (IC50 = 3.1 μg/mL) and the chloroquine-resistant (IC50 = 2.0 μg/mL) Indo strain of P. falciparum. G. laeve extracts were evaluated in the 4-day suppressive test in P. vinckei or P. berghei-infected mice and at doses of 50 mg/kg/day, these extracts suppressed parasitemias by 41 or 36 %, respectively, versus controls. Also, G. laeve extracts were toxic to rodents at doses of 100 mg/kg. There is a need for phytochemical studies and isolation of constituents to determine the antiplasmodial and toxic compounds present in G. laeve polar extracts (Muñoz et al., 2000).
In French Guiana, a study was performed on the traditional preparations used in the treatment of malaria. Thus, G. laeve bark (40 g) was placed in cold water (1 L), then boiled (15 min) and allowed to cool. In another preparation, G. laeve bark (40 g) was macerated in rum (250 mL) for 1 month).
G. argenteum bark was similarly macerated in rum. After total evaporation, G. laeve extract was found to be inactive (no schizonticidal activity) in vitro against chloroquine-resistant P. falciparum. This preparation inhibited parasitemia in vivo against Plasmodium yoelii versus controls by 35% at a dose of 23 mg/kg in rodents. G. argenteum extract was not tested in vitro. In vivo at a dose of 324 mg/kg this preparation inhibited P. yoelii by 44.3%. G. argenteum extracts were tested in the P. yoelii liver stage and exhibited 83% inhibition of the intra-hepatic cycle of the parasite, consistent with its use in malaria prophylaxis (Bertani et al., 2005).
In vitro antiplasmodial tests were performed on G. sericeum bark methanol-water extracts (90:10) and on isolated alkaloids (Steele et al., 2002). In these tests, the chloroquine-resistant K1 strain and chloroquine-sensitive T9-96 strain of P. falciparum were used. The crude extract exhibited IC50 = 1.78 ± 0.047 μg/mL against K1 strain. Geissoschizoline (4) and geissoschizoline N4-oxide (18) were inactive (IC50 > 40 μM) against both parasite strains. 1,2-de-hydrogeissoschizoline (19) was inactive against K1 and T9-96 P. falciparum strains (IC50 = 27.26 ± 10.9 and 35.37 ± 2.36 μM, respectively). Flavopereirine (6) exhibited IC50 = 11.53 ± 0.54 and 1.83 ± 0.10μM against K1 and T9-96 strains, respectively, and was the most active compound tested.
G. vellosii bark methanol extract exhibited high in vitro inhibitory activity (IC50 = 2.22 μg/mL) against the chloroquine-sensitive P. falciparum D10 strain. Geissospermine (1), geissoschizoline (4) geissolosimine (9) and geissoschizone (11) were isolated and exhibited a range of antiplasmodial activities (IC50 = 0.96 - 13.96 μM). Vellosiminol (8) was inactive (IC50 = 157 μM). Geissolosimine (9) exhibited the highest in vitro antiplasmodial activity (IC50 = 0.96 μM) (Mbeunkui et al., 2012).
The search for active substances in Amazonian medicinal plants is very promising, besides being a strategy for the discovery of new antimalarial drug leads (Andrade-Neto et al., 2007; Pohlit et al., 2013). As shown above, several Geissospermum alkaloids have high in vitro antiplasmodial activity. From in vivo work on extracts, it is clear that the antimalarial and toxic components of these extracts should be identified in future work. Thus, more work on the chemistry and antimalarial activity of Geissospermum species, especially on the in vivo antimalarial activity and toxicity of Geissospermum indole alkaloids is necessary. Future work should focus on addressing the dearth of in vivo studies on these alkaloids in animal models of malaria. Also, more compositional studies on G. argenteum, G. reticulatum and G. sericeum are needed The chemical composition of G. urceolatum has, to date, not been studied.
References
Almeida, M. R.; Lima, J. A.; Santos, N. P.; Pinto, A. C. 2009. Pereirina: o primeiro alcalóide isolado no Brasil? Brazilian Journal of Pharmacognosy, v 19, n. 4, p. 942-952. DOI: 10.1590/S0102-695X2009000600026.
Andrade-Neto, V. F.; Pohlit, A. M.; Pinto, A. C. S.; Silva, E. C. C.; Nogueira, K. L.; Melo, M. R. S.; Henrique, M. C.; Amorim, R. C. N.; Silva, L. F. R.; Costa, M. R. F.; Nunomura, R. C. S.; Nunomura, S. M.; Alecrim, W D.; Alecrim, M. G. C.; Chaves, F. C. M.; Vieira, P. P. R. 2007. In vitro inhibition of Plasmodium falciparum by substances isolated from Amazonian antimalarial plants. Memórias do Instituto Oswaldo Cruz, v 102, n. 3, p. 359-365. DOI: 10.1590/S0074-02762007000300016.
Araújo, J.Q.; Lima, J.A.; Pinto, A. C.; De Alencastro, R. B.; Albuquerque, M. G. 2011. Docking of the alkaloid geissospermine into acetylcholinesterase: A natural scaffold targeting the treatment of Alzheimer’s disease. Journal of Molecular Modeling, v 17, n. 6, p. 1401-1412. DOI: 10.1007/s00894-010-0841-2.
Ban, Y; Seo, M. 1961. The synthesis of β-carboline derivates-I. A synthesis of some 12H-indolo[2,3-a] pyridocolinum salts, including flavopereirine. Tetrahedron, v. 16, p. 5-10.
Barros J.S.; Oliveira, S. S.; Neves, S. M. B.; Tanae, M. M.; Souccar, C.; Lapa, A.J.; Landman, M. T R. L. 2006. Inibição da captação de serotonina por alcaloides de Geissospermum laeve Vell. Baill. XIX Simpósio de Plantas Medicinais do Brasil. Salvador, Brazil.
Bejar, O.; Goutarel, R.; Janot, M. M.; Le Hir, A. 1957. Constitution de la flavopéreirine, alcaloïde du Geissospermum laeve (Vellozo) Baillon (Apocynacées). Compt. Rend. Acad. Sci., v. 244, p. 2066-2068.
Beljanski, M. 1994. Flavopereirine-based pharmaceutical composition for treatment of HIV infection. WO9402146 A1, February 3, 1994.
Beljanski, M. 2005. Flavopereirine-based pharmaceutical composition and use thereof for treating HIV. Canadian Patent. CA 2120001 C (PCT/FR1993/000761), February 8, 2005.
Bemis, D.L.; Capodice, J.L.; Desai, M.; Katz, A.E.; Buttyan, R. 2009. β-carboline alkaloid-enriched extract from the Amazonian rain forest tree pao pereira suppresses prostate cancer cells. Journal of the Society for Integrative Oncology, v 7, n. 2, p. 59-65. DOI: 10.2310/7200.2009.0009.
Bertani, S.; Bourdy, G.; Landau, I.; Robinson, J.C.; Esterre, P.; Deharo, E. 2005. Evaluation of French Guiana traditional antimalarial remedies. Journal of Ethnopharmacology, v 98, p. 45-54. DOI: 10.1016/j. jep.2004.12.020.
Bertho, A.; Koll, M. 1961. Alkaloide der Pereiro-Rinde, VI. Die constitution von Pereirin. Chemische Berichte, v 94, p. 2737-2746. DOI: 10.1002/cber. 19610941022.
Block, K.I. 2014. In this issue [editorial]. Integrative Cancer Therapies, v 13, n. 3, p. 179-180. DOI: 10.1177/1534735414532011.
Brandão, M. D. G. L.; Cosenza, G. P.; Pereira, F. L. ; Vasconcelos, A. S.; Fagg, C. W. 2013. Changes in the trade in native medicinal plants in Brazilian public markets. Environmental Monitoring and Assessment, v 185, n. 8, p. 7013-7023. DOI: 10.1007/s10661-013-3081-y.
Brandão, M. G. L.; Grandi, T S. M.; Rocha, E. M. M. ; Sawyer, D. R.; Krettli, A. U. 1992. Survey of medicinal plants used as antimalarials in the Amazon. Journal of Ethnopharmacology, v 36, p. 175-182. DOI: 10.1016/0378-8741(92)90018-M.
Brito, A. R. M.; Brito, A. A. 1993. Forty years of Brazilian medicinal plant research. Journal of Ethnopharmacology, v. 39, p. 53-67. DOI: 10.1016/0378-8741(93)90050-F.
Camargo, M. R. M. 2011. Avaliação da atividade antimalárica e antimicrobiana de Geissospermum argenteum e Minquartia guianensis, coletadas em Roraima. Masters Dissertation. Federal University of Roraima. Roraima, Brazil.
Chang, C.; Zhao, W.; Xie, B.; Deng, Y.; Han, T; Cui, Y; Dai, Y; Zhang, Z.; Gao, J.; Guo, H.; Yan, J. 2014. Pao Pereira Extract Suppresses CastrationResistant Prostate Cancer Cell Growth, Survival, and Invasion Through Inhibition of NF-κB Signaling. Integrative Cancer Therapies, v. 13, n. 3, p. 249-258. DOI: 10.1177/1534735413510557.
Chiaroni, A.; Riche, C.; Pais, M.; Goutarel, R. 1976. Alcaloides indoliques CIV: Structure cristalline de la geissospermine. Tetrahedron Letters, v 17, n. 51, p. 4729-4730.
Chiaroni, A.; Riche, C. 1979. Structure et stéréochimie d’alcaloides indoliques. Structure de la geissospermine. Structural Cystallography and Crystal Chemistry, v B35, p. 1820-1825. DOI: 10.1107/ S0567740879007834.
Correia, A. F.; Segovia, J. F. O.; Gonçalves, M. C. A.; Oliveira, V. L.; Silveira, D.; Carvalho, J. C. T.; Kankazi, L. I. B. 2008. Amazonian plant crude extract screening for activity against multidrug-resistant bacteria. European Review for Medical and Pharmacology Sciences, v 12, n. 6, p. 369-380.
Forzza, R. C. (Org.). 2010. Catálogo de plantas e fungos do Brasil. Rio de Janeiro: Andrea Jakobsson Estúdio: Instituto de Pesquisa do Jardim Botânico do Rio de Janeiro, v 1.
Giorgetti, M.; Rossi, L.; Rodrigues, E. 2011. Brazilian plants with possible action on the central nervous system - a study of historical sources from the 16th to 19th century. Brazilian Journal of Pharmacognosy, v 21, n. 3, p. 537-555. DOI: 10.1590/S0102-695X2011005000044.
Gordillo-Román, B.; Reina, M.; Ruiz-Mesia, L.; Ruiz-Mesia, W.; Joseph-Nathan, P. 2013. Absolute configuration of indoline alkaloids from Geissospermum reticulatum. Tetrahedron Letters, v. 54, n. 13, p. 1693-1696. DOI: 10.1016/j.tetlet.2013.01.055.
Goutarel, R.; País, M.; Gottlieb, H.E.; Wenkert, E. 1978. 13C Analysis of geissospermine and its indole alkaloid monomer fragments. Tetrahedron Letters, v 14, p. 1235-1238. DOI: 10.1016/ S0040-4039(01)94510-1.
Hall, J. L.; Beljanski, S. P 2005. Flavopereirine and alstonine combinations in the treatment and prevention of prostate cancer. WO2005112964 (A1).
Henrique, M. C.; Nunomura, S. M.; Pohlit, A. M. 2010. Alcaloides indólicos das cascas de Aspidosperma vargasii e A. desmanthum. Química Nova, v. 33, n. 2, p. 2284-2287.
Hughes, N.A.; Rapoport, H. 1958. Flavopereirine, an alkaloid from Geissospermum vellosii. Journal of the American Chemical Society, v. 80, n. 7, p. 16041609. DOI: 10.1021/ja01540a024.
Ishiyama, H.; Matsumoto, M.; Sekiguchi, M.; Shigemori, H.; Ohsaki, A.; Kobayashi, J. 2005. Two new indole alkaloids from Aspidosperma subincanum and Geissospermum vellosii. Heterocycles, v. 66, n. 1, p. 651-658. DOI: 10.3987/COM-05-S(K)63.
Jácome, R. L. R. P.; Souza, R. A.; Oliveira, A. B. 2003. Comparação cromatográfica entre o extrato de Aspidospema parvifolium e o fitoterápico “pau-pereira”. Revista Brasileira de Farmacognosia, v. 13, p. 39-41. DOI: 10.1590/S0102-695X2003000300015.
Lima, J. A.; Costa, R. S.; Epifânio, R. A.; Castro, N. G.; Rocha, M. S.; Pinto, A. C. 2009. Geissospermum vellosii stembark: anticholinesterase activity and improvement of scopolamine-induced memory deficits. Pharmacology, Biochemistry and Behavior, v 92, p. 508-513. DOI: 10.1016/j.pbb.2009.01.024.
Lorenzi, H. 2002. Árvores brasileiras: manual de identificação e cultivo de plantas arbóreas do Brasil. 2. ed. Nova Odessa, SP: Instituto Plantarum. v 2.
Manske, R. H. F.; Harrison, W. A. 1965. Chapter 19 The alkaloids of Geissospermum species. Alkaloids: Chemistry and physiology, 8 (C), p. 679-691. DOI: 10.1016/S1876-0813(08)60058-5.
Mbeunkui, F.; Grace, M.H.; Lila, M.A. 2012. Isolation and structural elucidation of indole alkaloids from Geissospermum vellosii by mass spectrometry. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences, v 885-886, p. 83-89. DOI: 10.1016/j.jchromb.2011.12.018.
Mbeunkui, F.; Grace, M. H.; Lategan, C.; Smith, P.J.; Raskin, I.; Lila, M.A. 2012. In vitro anti-plasmodial activity of indole alkaloids from the stem bark of Geissospermum vellosii. Journal of Ethnopharmacology, v 139, p. 471- 477. DOI: 10.1016/j.jep.2011.11.036.
Milliken, W. 1997. Traditional antimalarial medicine in Roraima, Brazil. Economic Botany, v 51, p. 212-237. DOI: 10.1007/BF02862091.
Moore, R. E.; Rapoport, H. 1973. Geissovelline, a new alkaloid from Geissospermum vellosii. Journal of Organic Chemistry, v 38, n. 2, p. 215-230. DOI: 10.1021/jo00942a007.
Munoz, V.; Sauvain, M.; Bourdy, G.; Callapa, J.; Bergeron, S.; Rojas, I.; Bravo, J. A.; Balderrama, L.; Ortiz, B.; Gimenez, A.; Deharo, E. 2000. A search for natural bioactive compounds in Bolivia through a multidisciplinary approach. Part I. Evaluation of the antimalarial activity of plants used by Chacobo Indians. Journal of Ethnopharmacology, v 69, n. 2, p.127-137. DOI: 10.1016/S0378-8741(99)00148-8.
Oliveira, F. Q.; Junqueira, R. G.; Stehmann, J. R.; Brandão, M. G. L. 2003. Potential of medicinal plants as source of new antimalarials: species indicated in Brazilian ethnomedical bibliography. Revista Brasileira de Plantas Medicinais, v. 5, n. 2, p. 23-31.
Paccioni, J. P.; Husson., P. 1978. Alkaloids of Geissospermum argenteum (Apocynaceae). Phytochemistry, v 17, n. 12, p. 2146-2147.
Pohlit, A. M.; Lima, R. B. S.; Frausin, G.; Rocha e Silva, L. F. R.; Lopes, S. C.; Moraes, C. B.; Cravo, P.; Lacerda, M. V. G.; Siqueira, A. M.; Freitas-Junior, L. H.; Costa, F. T M. 2013. Amazonian plant natural products: perspectives for discovery of new antimalarial drug leads. Molecules, v 18, p. 9219-9240.
Puiseux, F.; Le Hir, A.; Goutarel, R.; Janot, M. M.; Lemen, J. 1959. Alkaloids of Geissospermum laeve. III. Geissoschizoline, apogeissoschizine and geissospermine. Annales Pharmaceutiques Françaises, v. 17, p. 626-633.
Rapoport, H.; Onak, T P.; Hughes, N. A.; Reinecke, M. G. 1958. Alkaloids of Geissospermum vellosii. Journal of the American Chemical Society, v 80, p. 1601-1604. DOI: 10.1021/ja01540a023.
Rapoport, H.; Windgassen Jr., R. J.; Hughes, N. A.; Onak, T P 1960. Alkaloids of Geissospermum vellosii. Futher studies on geissospermine and the structures of the indolic cleavage products, geissoschizine and apogeissoschizine. Journal of the American Chemical Society, v. 82, p. 4404-4414. DOI: 10.1021/ja01501a069.
Rapoport, H.; Moore, R. E. 1962. Alkaloids of Geissospermum vellosii. Isolation and structure determinations of vellosimine, vellosiminol and geissolosimine. Journal of Organic Chemistry, v 27, p. 2981-2985. DOI: 10.1021/jo01056a004.
Reina, M.; Ruiz-Mesia, W.; López-Rodríguez, M.; Ruiz-Mesia, L.; González-Coloma, A.; Martínez-Díaz, R. 2012. Indole alkaloids from Geissospermum reticulatum. Journal of Natural Products, v 75, n. 5, p. 928-934. DOI: 10.1021/np300067m.
Ribeiro, J. E. L. S.; Hopkins, M. J. G.; Vicentini, A.; Sothers, C.A.; Costa, M.A.S.; Brito, J.M.; Souza, M. A. D.; Martins, L. H. P.; Lohmann, L. G.; Assunção, P A. C. L.; Pereira, E. C.; Silva, C. F.; Mesquita, M. R.; Procópio, L. C. 1999. Flora da Reserva Ducke: guia de identificação das plantas vasculares de uma floresta de terra firme na Amazônia Central. Manaus: INPA-DFID, 798 p.
Santos, N. P.; Pinto, A. C.; Alencastro, R. B. 1998. Theodoro Peckolt: naturalista e farmacêutico do Brasil imperial. Química Nova, v 21, p. 666-670. DOI: 10.1590/S0100-40421998000500023.
Steele, J. C. P; Veitch, N. C.; Kite, G. C.; Simmonds, M. S. J.; Warhurst, D. 2002. Indole and β-carboline alkaloids from Geissospermum sericeum. Journal of Natural Products, v 65, p. 85-88. DOI: 10.1021/np0101705.
TPL - The Plant List. Version 1.1. URL: www.theplantlist.org/accessed on Sept. 8th, 2014.
Vigneron, M.; Deparis, X.; Deharo, E.; Bourdy, G. 2005. Antimalarial remedies in French Guiana: a knowledge attitudes and practices study. Journal of Ethnopharmacology, n. 98, p. 351-360. DOI: 10.1016/j.jep.2005.01.049.
WCSP. 2014. World Checklist of Selected Plant Families. Facilitated by the Royal Botanic Gardens, Kew. Published on the Internet; http://apps.kew.org/wcsp/ Retrieved September 8, 2014.
Wenkert, E.; Kilzer, J. 1962. A flavopereirine synthesis. Journal of Organic Chemistry, v. 27, p. 22832284. DOI: 10.1021/jo01053a552.
Werner, J. A. T.; Oliveira, S. M.; Martins, D. F.; Ferreira, J.; Santos, A.R.S. 2009. Evidence for a role of 5-HT1A receptor on antinociceptive action from Geissospermum vellosii. Journal of Ethnopharmacology, v 125, p. 163169. DOI: 10.1016/j.jep.2009.05.026.
Yu, J.; Chen, Q. 2014. The plant extract of pao pereira potentiates carboplatin effects against ovarian cancer. Pharmaceutical Biology, v 52, n. 1, p. 36-43. DOI: 10.3109/13880209.2013.808232.