ETNOFARMACOLOGIA
Biological activity and quassinoid content of fruits from the amazonian medicinal plant Picrolemma sprucei (Simaroubaceae)
Atividade biológica e teor de quassinoides dos frutos da planta medicinal amazônica Picrolemma sprucei (Simaroubaceae)
Abstract
Picrolemma sprucei Hook. f. (Simaroubaceae) is a medicinal plant that is used throughout the Amazon region in the treatment of malaria. No previous report has been published on the biological activity or chemical composition of the fruit of this species. In the present study, antimalarial effects of the fruit from P. sprucei was evaluated. Furthermore, a rapid and simple thin-layer chromatography-photodensitometry (TLC-PD) analytical method is utilized for the semi-quantitative determination of the quassinoids isobrucein B (1) and neosergeolide (2) in P. sprucei fruit. EtOH extracts of the whole fruit, pericarp and seeds (pits) of P. sprucei exhibited high in vitro inhibitory activity (IC50 = 0.6, 1.5 and 0.2 mg/mL, respectively) against the K1 strain of the human malaria parasite Plasmodium falciparum. Hex, CHCl3, EtOAc, BuOH and MeOH-H2O fractions obtained by liquid-liquid partitioning of seed and pericarp EtOH extracts provided seed (IC50 = 0.01-3.7 mg/mL) and pericarp (IC50 = 0.01-9.1 mg/mL) fractions exhibiting high inhibitory activity against P. falciparum. CHCl3 fractions had the highest in vitro antimalarial activities (IC50 = 10 ng/mL), probably due the high quassinoid content. The CHCl3 fraction of the seed extract exhibited 39.8 % (m/m) of 1 and 3.5% (m/m) of 2 while the CHCl3 fraction of the pericarp extract contained 5.0 % (m/m) of each quassinoids. The antiplasmodial activity of the fractions was due presumably to quassinoid and non-quassinoid active components. P. sprucei fruit pulp and pit have potential as phytotherapeutic agents.
- Keywords:
- Simaroubaceae.
- caferana.
- neosergeolide.
- isobrucein B.
- Plasmodium falciparum.
Resumo
Picrolemma sprucei Hook. f. (Simaroubaceae) é uma planta medicinal utilizada por toda a região Amazônica no tratamento da malária. Nenhum trabalho anterior foi publicado sobre a atividade biológica ou composição química dos frutos desta espécie. No presente estudo, a atividade antimalárica dos frutos de P. sprucei foi avaliada. Além disso, um método analítico simples e rápido baseado em cromatografia em camada delgada-fotodensitometria (CCD-FD) é utilizado para a determinação semi-quantitativa do teor de quassinóides isobruceina B (1) e neosergeolida (2) nos frutos de P. sprucei. Extratos etanólicos do fruto inteiro, pericarpo e sementes (caroços) de P. sprucei exibiram elevada atividade in vitro (CI50 = 0,6, 1,5 e 0,2 mg/mL, respectivamente) frente à cepa K1 do parasita da malária humana Plasmodium falciparum. Frações obtidas em hex, CHCl3, AcOEt, BuOH e MeOH-H2O, por partição liquid-líquido dos extratos etanólicos das sementes e pericarpos, também proveram elevada atividade antimalárica (CI50 = 0,01-3,7 mg/mL para frações obtidas das sementes e CI50 = 0,01-9,1 mg/mL para frações obtidas dos pericarpos). As frações clorofórmicas apresentaram as maiores atividades antimaláricas (CI50 = 10 ng/mL), provavelmente devido ao teor elevado de quassinóides. A fração clorofórmica do extrato da semente exibiu 39,8 % (m/m) de 1 e 3,5% (m/m) de 2 enquanto a fração clorofórmica do extrato do pericarpo continha 5,0 % (m/m) de cada quassinóide. A atividade antiplasmodial das frações foi presumivelmente devida à presença de quassinóides e não-quassinóides ativos. A polpa da fruta e o caroço tem potencial como agentes fitoterápicos.
- Palavras-Chave:
- Simaroubaceae.
- caferana.
- neosergeolida.
- isobruceina B.
- Plasmodium falciparum.
Introduction
According to the World Health Organization (WHO), about 3.3 billion people live in regions where there is a risk of malaria infection. At least 219 million people are infected by malaria parasite every year causing an estimated 660.000 deaths. The economic costs of malaria are also high and it is estimated that annual economic growth is diminished by as much as 1.3% in poor and developing countries where malaria transmission is intense as is the case in Brazil (WHO, 2012). Today, with the exception of a few countries in Central America, countries having high incidences of malaria in general must also deal with increasing resistance of malaria parasites to antimalarial drugs. A decrease in sensitivity of several strains of Plasmodium falciparum to artemisinin in vitro was observed in China and Vietnam (Dondorp et al., 2009). These facts are of concern especially in poor regions with high incidence of malaria as is the case of the Brazilian Amazon where human malaria is endemic. In this region of dense forest and high biodiversity, plants with medicinal potential are intensively used by the local population to treat malaria (Milliken, 1997).
The genus Picrolemma (Simaroubaceae) is composed of only two species-P. huberi (syn. Cedronia granatensis Cuatr. and syn. P. granatensis Cuatr.) and P. sprucei Hook. f. (syn. P. pseudocoffea Ducke). Both species are native to and found exclusively in the Amazon region (Cronquist, 1944). Know in the Brazilian Amazon by the common name "caferana", P. sprucei is used in traditional medicine in Peru, Brazil and French Guyana to treat malaria (Milliken, 1997; Silva et al., 2009). Our research group in collaboration with others has demonstrated the antimalarial (Andrade-Neto et al., 2007), anthelmintic (Nunomura et al., 2006), larvicidal (Pohlit et al., 2004), cytotoxic (Quignard et al., 2004; Silva et al., 2009, Cavalcanti et al., 2012) and antitumor (Silva et al., 2009, Cavalcanti et al., 2012) activities of caferana stem and root extracts, the isolated quassinoid components isobrucein B (1) and neosergeolide (2) (figure 1) found in this plant and semi-synthetic derivatives of 1 and 2 (Amorim and Pohlit, 2006). Agronomic studies towards the domestication of caferana and improving the process of obtaining this plant in a sustainable fashion are under development to further allow the use of this plant as a therapeutic agent (Silva, Amorim and Pohlit, 2009). There are no data in the literature on the chemical composition or biological activity of P. sprucei fruit. The aim of the present work was to evaluate the antimalarial potential and chemical composition of extracts and fractions of the fruit of P. sprucei.
Materials and methods
Plant materials, preparation of extracts and fractions: Collection was performed in Manaus, Amazonas State, Brazil in the period February-April, 2006. Voucher specimens were deposited at the INPA Herbarium (INPA 223883). Dried whole fruit (191.26 g), pericarp (16.29 g) and dried seeds (18.69 g) were separately ground in a mincer and macerated in EtOH (100 mL, 3 x 1 week). The filtrates from these extractions were evaporated to dryness under vacuum. Each extract was dissolved in a mixture of MeOH (45 mL) and H2O (5 mL) (9:1) and partitioned sequentially with Hex, CHCl3, EtOAc and n-BuOH (3 x 10 mL of each solvent). These fractions were evaporated to dryness under vacuum. The procedure used for the isolation of 1 and 2 (figure 1) from the roots and stems of P. sprucei has been described previously (Silva, Amorim and Pohlit, 2009).

TLC-photodensitometric determination of 1 and 2 in extracts and fractions: Extracts and fractions of P. sprucei fruit were first analyzed qualitatively for the presence of 1 and 2 by normal-phase thin-layer chromatography (TLC) (20 x 20 cm Merck F254 plates (Merck, Darmstadt, Germany) which were cut to obtain 10 x 20 cm plates). Et2O and i-PrOH (9:1) were used as mobile phase. Quassinoids 1 and 2 were semi-quantified in extracts and fractions by external calibration TLC-photodensitometry on a Hewlett-Packard Image Scanner III high resolution optical scanner using GE Healthcare Lab Scan software version 6.0 (Uppsala, Sweden). Spots were visualized qualitatively for the presence or absence of detectable quassinoids using an ultraviolet (UV) lamp (254 nm) and compared to spots corresponding to 1 and 2. For quantification, TLC plates were conveniently developed by spraying with phosphomolybdic acid (Merck, Darmstadt, Germany) solution (20.0 g of phosphomolybdic acid in 100 mL of ethyl alcohol followed by filtering) then spraying with p-anisaldehyde (Merck, Darmstadt, Germany) solution (0.5 mL p-anisaldehyde, 50 mL of glacial CH3CO2H, and 1.0 mL conc. H2SO4). The RF values for 1 and 2 were 0.6 and 0.4, respectively, under these conditions. The limits of quantification (LOQ) for 1 and 2 by TLC-densitometry in the extracts and fractions of P. sprucei fruit were 0.08 and 0.12 μg, respectively. For qualitative analyses performed with illumination of TLC F254 plates with UV lamp (254 and 366 nm) the limit of detection (LOD) was 0.03 μg for 1 and 0.06 μg for 2.
Parasite culture and in vitro antimalarial tests: Chloroquine-resistant P. falciparum strain K1 was acquired from MR4 (Malaria Research and Reference Reagent Resource Center, Manassas, Virginia, USA) and was used in the in vitro tests. Parasites were maintained in continuous culture in A+ human erythrocytes using RPMI medium supplemented with 10 % human serum (Trager and Jensen, 1976). The antiparasitic effect of the extracts and fractions was measured by growth inhibition percentage (Carvalho and Krettli, 1991). Trophozoite-stages in sorbitol-synchronized blood (Lambros and Vanderberg, 1979) were cultured at 1 % parasitaemia and 2.0 % hematocrit and then incubated with samples, diluted with DMSO (≤ 0.02 % final concentration) in culture medium (RPMI 1640) for 24 h at 37 °C. The antiparasitic effect was measured using an optical microscope.
Positive control wells containing the reference anti-malarial drug chloroquine in standard concentrations (Rieckmann et al., 1978; WHO, 2001) were used for each experiment. The stock solutions were further diluted with complete medium (RPMI 1640 plus 10 % human serum) to each of the concentrations used (100 to 0.0001 mg/mL in seven dilutions). The half-maximal inhibitory (IC50) responses as compared to the drug-free controls were estimated by interpolation using Microcal OriginÒ software. Each duplicate experiment was repeated three times and blood smears were read blind (Rieckmann et al. 1978).
Results and Discussion
The yields (as a percentage of extracted plant material) of whole fruit, pericarp and seed EtOH extracts were 12.0, 8.2 and 5.8 % m/m, respectively, based on extracted plant materials. 1 and 2 could be detected in whole fruit, pericarp and seed extracts by UV illumination of TLC plates after elution. All EtOH extracts contained comparable amounts of the quassinoids 1 and 2 according to TLC-PD analysis (table 1). The order of decreasing antiplasmodial activity among the EtOH extracts was pit > whole fruit > pericarp.
| Part | Extract/fraction | Quassinoid Content (%) | P falciparum (IC50 pg/mL) |
|
| 1 | 2 | |||
| whole fruit | EtOH extract | 0.2-0.5 | 0.4-0.8 | 0.6 |
| pericarp | EtOH extract | 0.2-0.5 | 0.4-0.8 | 1.5 |
| Hex fraction | < 0.2 | < 0.4 | 9.1 | |
| CHCl3 fraction | 5.0 | 5.0 | 0.01 | |
| EtOAc fraction | < 0.2 | < 0.4 | 0.2 | |
| n-BuOH fraction | < 0.2 | < 0.4 | 1.1 | |
| MeOH-H2O fraction | < 0.2 | < 0.4 | 0.6 | |
| pit | EtOH extract | 0.2-0.5 | 0.4-0.8 | 0.2 |
| Hex fraction | < 0.2 | < 0.4 | 3.7 | |
| CHCl3 fraction | 39.8 | 3.5 | 0.01 | |
| EtOAc fraction | < 0.2 | < 0.4 | 0.1 | |
| n-BuOH fraction | < 0.2 | < 0.4 | 0.3 | |
| MeOH-H2O fraction | < 0.2 | < 0.4 | 0.1 | |
| chloroquine | 0.03 | |||
| 1 | 0.003 | |||
| 2 | 0.002 | |||
Liquid-liquid was successful at concentrating quassinoids and antiplasmodial activity into the fractions. CHCl3 fractions obtained from pericarp and seed extracts presented yields of 14.3 and 15.9 % m/m, respectively, based on the mass of fraction obtained from the mass of extract that was partitioned. The CHCl3 fraction of the seed extract exhibited a significant quantity of 1 (39.8 %, m/m) and also 2 (3.5 %, m/m) while the CHCl3 fraction of the pericarp extract contained 5.0 % of each of these quassinoids (table 1). Other fractions (hex, EtOAc, n-BuOH and MeOH-H2O) obtained during partitioning did not exhibit appreciable amounts of quassinoids 1 and 2 according to TLC analysis. In general, all seed and pericarp extracts and fractions exhibited significant in vitro inhibition of the K1 strain of P. falciparum used in tests. Quassinoid-rich CHCl3 fractions derived from fruit extracts were the most active inhibitors of P. falciparum. The antimalarial activity of both CHCl3 fractions was comparable to that of chloroquine and about an order of magnitude less than that of pure 1 or 2. High antiplasmodial activity (low IC50 values) was exhibited by several fractions which did not contain detectable amounts of quassinoids. This observation is consistent with the notion that there are other antimalarial components in P. sprucei fruit besides 1 and 2. The presence of non-quassinoid-related antiplasmodial activity has not been reported previously for P. sprucei and should be investigated in future work.
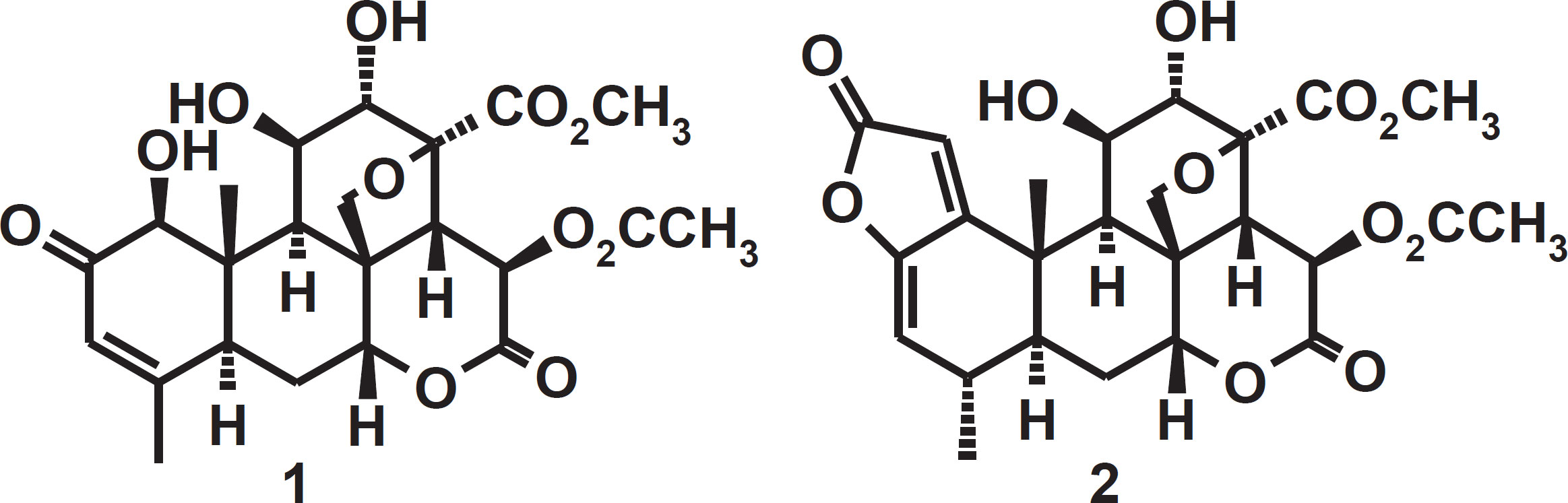
P. sprucei fruit extracts and fractions may be a good alternative for standardization of phytotherapeutic drugs, especially extracts containing quassinoids. It is possible that non-quassinoid compounds contribute to antimalarial activity in traditional remedies prepared from P. sprucei and/or modulate toxic effects associated with isolated quassinoids. In addition, standardized herbal remedies may be better tolerated and exhibit low or moderate toxicity. As shown before, quassinoids present in P. sprucei have strong antimalarial activity in vivo against P. berghei infection in mice at a very low dose (DE50 0.26 mg/kg/day) (Fandeur, Moretti and Polonsky, 1985).
Acknowledgements
This research was financially supported by the CNPq (Projects Ataca-Malaria No: 550260/01-3, PPG-7 No: 557106/2005-2, PRONEX Rede Malaria No: 555665/2009-7, and No: 557106/2005-2, Bionorte No: 554317/2010-9). Authors recognize scholarships received from the agencies indicated: MRSM (CNPq 142115/06), RCNA (CNPq 142115/06-1) and LFRS (FAPEAM).
References
Amorim, R.N.; Pohlit, A.M. 2006. Picrolemma sprucei Hook. f.: uso tradicional, princípios ativos e seus derivados semi-sintéticos, exploração comercial e econômica. Revista Fitos, v. 2, n. 1, p. 19-26.
Andrade-Neto V.F.; Pohlit, A.M.; Pinto, A.C.S.; Silva, E.C.C.; Nogueira, K.L.; Melo, M.R.S.; Henrique, M.C.; Amorim, R.C.N.; Silva, L.F.R.; Costa, M.R.F.; Nunomura, R.C.S.; Nunomura, S.M.; Alecrim, W.D.; Alecrim, M.G.C.; Chaves, F.C.M.; Vieira, P.P.R. 2007. In vitro inhibition of Plasmodium falciparum by substances isolated from Amazonian antimalarial plants. Memórias do Instituto Oswaldo Cruz, v 102, n. 3, p. 359-365.
Cavalcanti, B.C.; Costa, P.M.; Carvalho, A.A.; Rodrigues, F.A.R.; Amorim, R.C.N.; Silva, E.C.C.; Pohlit, A.M.; Costa-Lotufo, L.; Moraes, M.O.; Pessoa, C. 2012. Neosergeolide-induced apoptosis in human leukemia HL-60 cells Involvement of intrinsic mitochondrial pathway in neosergeolide induced apoptosis of human HL-60 leukemia cells: the role of mitochondrial permeability transition pore and DNA damage. Pharmaceutical Biology, v 50, n. 8, p. 980-993.
Carvalho, L.H.; Krettli, A.U. 1991. Antimalarial chemotherapy with natural products and chemically defined molecules. Memórias do Instituto Oswaldo Cruz, v 86, n. 2, p. 181-184.
Cronquist, A. 1994. Studies in the Simaroubaceae - IV. Resume of the American genera Brittonia, v 5, n. 2, p. 128-47.
Dondorp, A.M.; François, N.; Poravuth, Y.; Debashish, D.; Aung, P.; Tarning, J.; Khin, M.L. 2009. Artemisinin resistance in Plasmodium falciparum malaria. New England Journal of Medicine, v. 361, p. 455-467.
Fandeur, T.; Moretti, C.; Polonsky, J. 1985. In Vitro and In Vivo assessment of the antimalarial activity of sergeolide. Planta Medica, v 51, p. 20-23.
Lambros, C.; Vanderberg, J.P. 1979. Syncronization of Plasmodium falciparum erythrocytic stages in culture. Journal of Parasitology, v 65, n. 3, p. 418-420.
Milliken,W 1997. Plants for malaria. Plants for fever: Medicinal species in Latin America - a bibliographic survey, Kew Publishing, Kew, United Kingdom
Nunomura, R.C.S.; Silva, E.C.C.; Oliveira, D.F.; Garcia, A.M.; Boeloni, J.N.; Nunomura, S.M.; Pohlit, A.M. 2006. In vitro studies of the anthelmintic activity of Picrolemma sprucei Hook. f. (Simaroubaceae). Acta Amazonica, v. 36, n. 3, p. 327-330.
Pohlit, A.M.; Quignard, E.L.J.; Nunomura, S.M.; Tadei, W.P.; Hidalgo, A.F.; Pinto, A.C.S.; Santos, E.V.M.; Morais, S.K.R.; Saraiva, R.C.G.; Ming, L.C.; Alecrimm, A.M.; Ferraz, A.B.; Pedroso, A.C.S.; Diniz, E.V.; Finney, E.K.; Gomes, E.O.; Dias, H.B.; Souza, K. S.; Oliveira, L.C.P.; Don, L.C.; Queiroz, M.M.A.; Henrique, M.C.; Santos, M.; Lacerda Júnior, O.S.; Pinto, P.S.; Silva, S.G.; Graça, Y.R. 2004. Screening of plants found in the State of Amazonas, Brazil for larvicidal activity against Aedes aegypti larvae. Acta Amazonica, v 34, n. 1, p. 97-105.
Quignard, E.L.J.; Nunomura, S.M.; Pohlit, A.M.; Alecrim, A.M.; Pinto, A.C.S.; Portela, C.N.; Oliveira, L. C.P.; Don, L.C.; Silva, L.F.R.; Henrique, M.C.; Santos, M.; Pinto, P.S.; Silva, S.G. 2004. Medial lethal concentrations of Amazonian plant extracts in the brine shrimp assay. Pharmaceutical Biology, v 42, n. 3, p. 253-257.
Rieckmann, K.H.; Sax, L.J.; Campbell, G.H.; Mrema, J.E. 1978. Drug sensitivity of Plasmodium falciparum. An in vitro microtechinique. Lancet, v 1, p. 22-23.
Silva, E.C.C.; Amorim, R.C.N.; Pohlit, A.M. 2009. Gram-scale isolation of isobrucein B and neosergeolide from Picrolemma sprucei Hook. f. Acta Amazonica, v 39, n. 1, p. 229-232.
Silva, E.C.C.; Cavalcanti, B.C.; Amorim, R.C.N.; Lucena, J.F.; Quadros, D.S.; Tadei, W.P.; Montenegro, R.C.; Costa-Lotufo, L.V.; Pessoa, C.; Moraes, M.O.; Nunomura, R.C.S.; Nunomura, R.C.S.; Melo, M.R.S.; Andrade-Neto, V.F.; Silva, L.F.R.; Vieira, P.P.R.; Pohlit, A.M. 2009. Biological activity of neosergeolide and isobrucein B (and two semi-synthetic derivatives) isolated from the Amazonian medicinal plant Picrolemma sprucei (Simaroubaceae). Memórias do Instituto Oswaldo Cruz, v 104, n. 1, p. 48-55.
Trager, W.; Jensen, J.B. 1976. Human malaria parasites in continuous culture. Science, v. 193, n. 4254, p. 673-675.
World Health Organization (WHO). 2001. In vitro micro-test (Mark III) for the assessment of the response of Plasmodium falciparum to chloroquine, mefloquine, quinine, amodiaquine, sulfadoxine/pyrimetamine and artemisinin. Division of Control of Tropical Disease, Rev. 2 CTD/MAL /97.20.
World Health Organization (WHO). 2012. World Malaria Report. Available from: http://www.who.int/malaria/publications/world_malaria_report_2012/wmr2012_factsheet.pdf.