QUÍMICA
Study of anti-microbial and anti-pectinase activity of extracts and compounds isolated from Siphoneugena densiflora (Myrtaceae) and Vitex polygama (Lamiaceae)
Estudo das atividades antimicrobial e inibidora de pectinases dos extratos e compostos isolados de Siphoneugena densiflora (Myrtaceae) e Vitex polygama (Lamiaceae)
Abstract
Crude hydroalcoholic extracts (HA) and twelve compounds isolated from Siphoneugena densiflora O. Berg and Vitex polygama Cham. were tested in vitro as growth inhibitors of the fungus Leucoagaricus gongylophorus (Singer) Möller, that lives in symbiosis with the leaf-cutting ant Atta sexdens, as well as inhibitors of the fungus' pectinases. Also, the effects of the extracts and the compounds were assessed in a panel of bacteria and yeasts similar to those living in symbiosis with the ant. HA extracts from S. densiflora displayed significant antimicrobial results, mainly against Pseudomonas aeruginosa. Caffeoyl 6-O-β-D-glucopyranoside was the most promising compound in the control of L. gongylophorus growth.
- Palavras-chave:
- Leucoagaricus gongylophorus.
- taninos hidrolisáveis.
- Atta sexdens rubropilosa.
- ensaios antimicrobianos.
- atividade inibitória da enzima pectinase.
Resumo
Extratos hidroalcoólicos (HA) e doze substâncias naturais isoladas de Siphoneugena densiflora O. Berg and Vitex polygama Cham. foram testadas in vitro como inibidores de crescimento do fungo Leucoagaricus gongylophorus (Singer) Möller, simbionte da formiga cortadeira Atta sexdens, e de suas pectinases. Os efeitos dos extratos e compostos também foram avaliados sobre várias bactérias e leveduras similares às espécies que vivem em simbiose com a formiga. Os extratos HA de S. densiflora exibiram os resultados antimicrobianos mais significativos, principalmente contra a Pseudomonas aeruginosa. O 6-O-β-D-glucopiranosídeo de cafeoila foi o composto mais promissor no controle do crescimento do L. gongylophorus.
- Keywords:
- Leucoagaricus gongylophorus.
- hydrolysable tannins.
- Atta sexdens rubropilosa.
- antimicrobial assays.
- pectinases inhibition activity.
Introduction
Leaf-cutting ants from Atta genus are regarded as important damaging agriculture pests in South America. Atta sexdens rubropilosa Forel (Hymenoptera: Formicidae), one of the most ordinary species of Atta, lives in obligate symbiosis with the basidiomycete Leucoagaricus gongylophorus (Singer) Möller and in association with several other microorganisms, for example the bacterium Burkolderia sp. and yeasts such as Candida, Cryptococcus and Trichosporon (Carreiro et al., 1997; Santos et al., 2004; Erthal et al., 2009). The basidiomycete provides the ants with nutrients (Martin and Weber, 1969) and plant polysaccharide degrading enzymes (Silva et al., 2006), like the pectinases, while the ants supply the symbiotic fungus with a variety of substrates (Weber, 1972) and also stimulate its growth on plant material inside their nests, in chambers called "fungus garden" (Martin et al., 1975). The rupture of that symbiosis either by the employment of antimicrobial agents or by the use of pectinases activity inhibitors can contribute to controlling the insects in the field.
As part of our search for plant secondary metabolites to be used as insecticides and antibiotics (Gallo et al., 2006a; Cazal et al., 2009; Bicalho et al. 2012), hydroalcoholic extracts and isolated compounds from Siphoneugena densiflora O. Berg (Myrtaceae) and Vitex polygama Cham. (Lamiaceae) were tested against several microbes, on L. gongylophorus growth, and as inhibitors of the fungal pectinases activity. Chemical interactions between the test compounds and the reagents of the enzymatic assay were evaluated as well.
Materials and Methods
Plant material and extraction procedures
V. polygama and S. densiflora were collected in July 2000, in the city of Poços de Caldas, Minas Gerais, Brazil. Voucher specimens were deposited at the Herbarium of the Faculdade de Filosofia, Ciências e Letras of the University of São Paulo (SPFR), campus Ribeirão Preto, under the acquisition number 9968, and at the Herbarium of the Department of Botany of University of São Paulo, São Paulo, Brazil, respectively.
The extracts and the isolated compounds (1 to 12) were obtained and identified according to procedures formerly described (Gallo et al. 2006a; Gallo et al. 2006b; Gallo et al., 2008).
Antimicrobial assay
Antimicrobial activity of extracts was determined using the paper disk diffusion method (Bauer et al., 1966). The following bacterial strains: Staphylococcus aureus (ATCC 6538), Bacillus cereus (CCT 1436), Pseudomonas aeruginosa (ATCC 15442), Escherichia coli (CCT 1457), Micrococcus roseus (CCT 1469), and fungal strains: Candida albicans (RJ/500008), Cryptococcus laurentii (RJ/50359), Saccharomyces cerevisiae (CCT0758) and Trichosporon cutaneum (UCD121) were used in the experiments. Tetracycline, for bacteria, and nystatin, for fungi, were used as positive controls. Minimal inhibitory concentration (MIC) values were determined as the lowest concentration either of extracts or standard compounds that completely inhibited macroscopic microorganism growth by a microplate Alamar Blue assay-MABA (Salvat et al., 2001).
Pectinases inhibition assay
The enzymatic inhibitory assay was carried out using a Phoenix AP 56 glass tube shaker, Eppendorf Centrifuge 5417 R (Germany), Beckman DU 640 spectrometer, UV-1650 PC/SHIMADZU spectrometer, CPS-Controler/ SHIMADZU (Japan).
In order to harvest the fungal pectinases, the worker ants of A. sexdens rubropilosa were placed in a frozen chamber at -15oC for 10 minutes to be anesthetized. Subsequently, each ant had its abdomen softly squeezed by calipers to release the inside liquid, which was collected with a Pasteur capillary pipette and transferred to a flask containing 50 mM sodium phosphate buffer at pH 6. At the end of the harvest, the obtained solution was diluted with deionized water by 1:500 (v/v) and named faecal fluid whose composition was mainly of fungal pectinases.
The enzymatic inhibitory activity of extracts and isolated compounds was measured as following: 4 µL of solution of the plant extract or the isolated compound (50 µg/µL in DMSO) were added to 150 µL of pectin solution (2.0 g in100 mL of 50 mM sodium citrate/ phosphate buffer at pH 5), 75 µL of faecal fluid and 71 µL of deionized water. For the solvent control, 4 µL of DMSO was used. In addition, an experiment wi thout solvent or extract was conducted, using 150 µL of deionized water and 150 µL of pectin solution. The experiment was carried out in duplicate; the reaction mixture was incubated at 37 °C and shaken for 30 minutes. Aliquots of 50 µL of each above mentioned mixture were collected during the initial and the final incubation time, and were added to 100 µL of DNSA reagent (3,5-dinitrosalicilic acid; Miller, 1959) and 100 µL of deionized water. The resultant solutions were boiled during 5 min, followed by ice-refrige rating and centrifuging. The optical density (OD) of the collected supernatant was measured at 540 nm. The pectinases inhibition activity (I) was calculated through the formula I = [1 - (TC-1)] x 100, where T and C are the pectinases activity, obtained as OD values, in the presence and in the absence of a given plant extract or compound, respectively.
The influence of the tested tannins on the assay reagents was assessed by mixing the tannins separately with each one of the biochemical assay reagents, and the procedure for OD measurement was carried out as above mentioned.
Differences between treatment means were performed by Mann Whitney or Student's t tests (Snedecor and Cochran, 1989). Results are given in the text as probability values, with P < 0.05 adopted as the criterion of significance.
L. gongylophorus growth inhibition
The fungus was isolated from an A. sexdens rubropilosa nest and kept in culture media (Pagnocca et al., 1990). Compounds and extracts were tested against L. gongylophorus growth according to established protocols (Bigi et al., 2004). Hydroalcoholic extracts of twigs (SD-HAT), stem (SD-HAS), leaves (SD-HAL) and root bark (SD-HAR) from S. densiflora were tested at 1000 µg/mL. Isolated substances were tested at 50 or 100 µg/mL. The assays were run twice (two sets of five tubes for each concentration). Fungal growth was estimated macroscopically on the basis of mycelia surface and density after 30 days of incubation and the modal value among the five replicates was registered.
Results and Discussion
Isolation and structure elucidation of compounds
Compounds 1 to 12 (FIGURE 1) were identified by comparison of their NMR, MS, UV, and IR spectros copic data with data previously reported in literature as following:
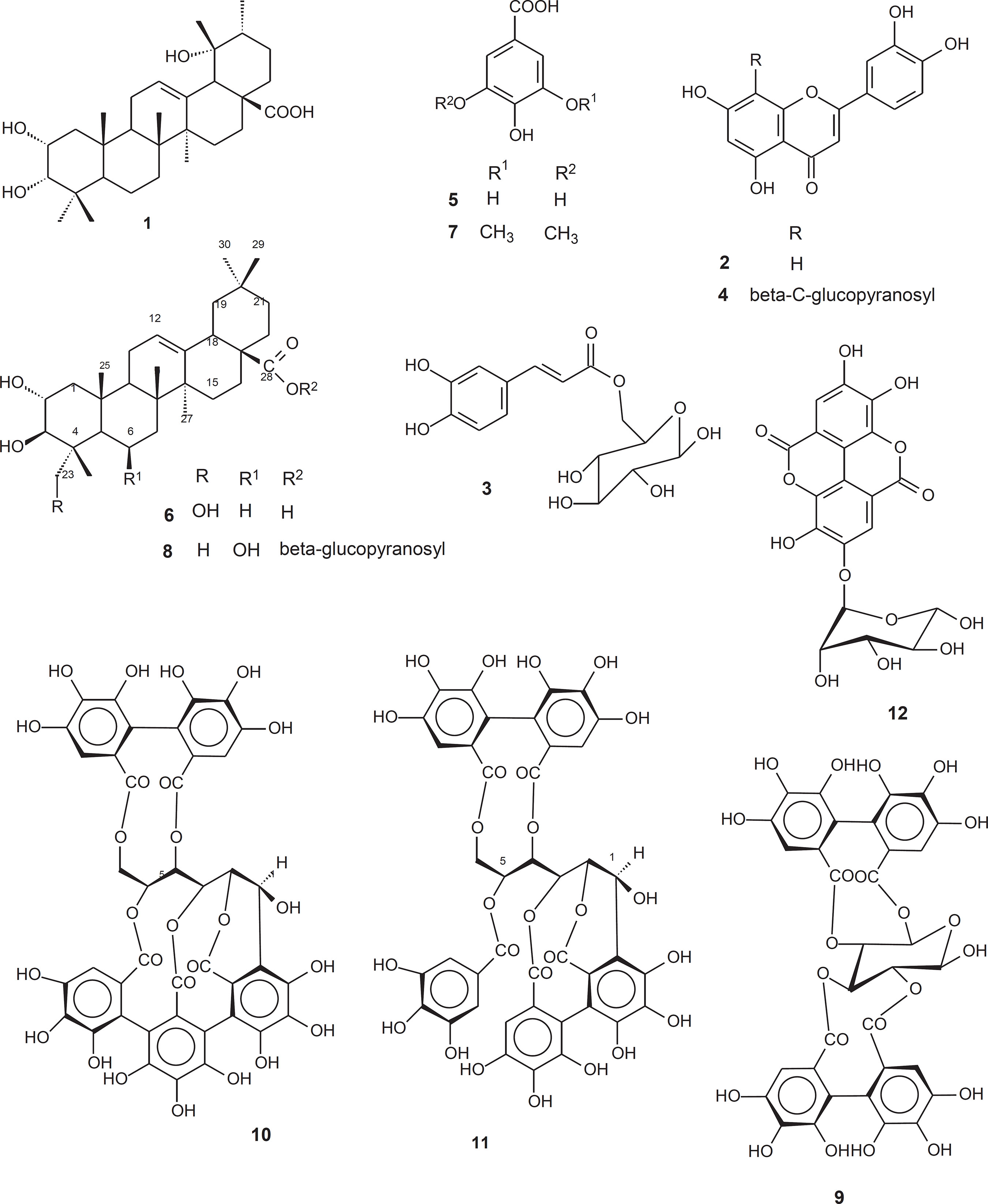
2α,3α,19α-trihydroxyurs-12-en-28-oic acid or eusca- fic acid (1) (Mahato and Kundu, 1994), luteolin (2) (Agrawal, 1989), caffeoyl 6-O-β-D-glucopyranoside (3) (Shimomura, Sashida and Adachi, 1988), orientin (4) (Dayrit et al., 1987), gallic acid (5) (Tiang et al., 2000), arjunolic acid (6) (Mahato and Kundu, 1994), syringic acid (7) (Hung and Yeng, 2002), 28-β-D-glucopyronosyl-6β-hydroxymaslinate (8) (Gallo et al., 2006b), β-pedunculagin (9) (Okuda et al., 1983), castalagin (10), casuarinin (11), and ellagic acid 4-O-α-L-rhamnopyranoside (12) (Gallo et al., 2006a).
Antimicrobial assay
TABLE 1 summarizes the obtained results when HA extracts from S. densiflora were assessed against some yeast, fungus and bacterium species through the disk diffusion method (DDM). The extract SD-HAL displayed potent activities against Pseudomonas aeruginosa and Micrococcus roseus, causing an inhibition zone diameter (IZD) of 11 mm on both species, in comparison to those exhibited by the standard drug (IZD of 10 and 12 mm, respectively). In addition, the other extracts that showed IZD higher than 8 mm on the tested bacteria and fungus species were considered active, albeit they caused no effect toward the yeasts development. The antibacterial activity of HA extracts was corroborated by the determination of the minimal inhibitory concentration values (MIC, TABLE 2). The Gram-negative P. aeruginosa, known as one of the most prevalent causes of nosocomial infection in the world, was found to be particularly susceptible to the HA extracts (MIC < 31.3 µg/mL), indicating them as promising sources of antibiotic compounds against that species. The antimicrobial activity of some of the isolated compounds, 1 to 8, 10 and 11, was also evaluated by DDM. They showed no effect against the microorganisms when tested at 50 µg/disk (data not shown), except for gallic acid (5), which presented an IZD of 15 mm on Staphylococcus aureus growth. In general, flavonoids, triterpenes and hydrolysable tannins, the more representative classes of the compounds tested, are known for displaying antimicrobial activities (Serrano et al., 2009; Buzzini et al., 2008; Harborne and Williams, 2000; Li et al., 2002). For example, the flavone luteolin (2) performed strong activity against some filamentous fungi, presenting IC50 values from 38 to 81 µg/mL (Wang et al., 2010). Flavone 2 is also known as an effective phytoalexin in sorghum seeds (Du et al., 2010), acting as a competitive inhibitor of enzymes in the biosynthesis of melanin, a fungal virulence factor (Brunskole et al., 2009). Conversely, luteolin caused no effect against twenty microbe strains when tested at 2.5 mg/mL (Schinor et al., 2007). The phenolic acid 5 showed an IZD of 7 mm on S. aureus and C. albicans growth when tested at 100 µg/disk (Fogliani et al., 2005). Tannins 9, 10 and 11 were effective against some Vibrio and Aeromonas species showing MIC around 83 µg/mL (Yanamaka et al., 2008), while castalagin (10) presented MIC values of 114 and 705 µg/mL on S. aureus (ATCC 29213) and Escherichia coli (IFO 3972, ATCC 25922), respectively (Taguri, Tanaka and Kouno, 2004). The triterpene arjunolic acid (6) was found to be active against E. coli (NEU 1006; IC50 of 3 µg/spot) (Djoukeng et al., 2005) as well C. albicans (ATCC 90028; MIC of 50 µg/mL) (Bisoli et al., 2008), and its antifungal activity was highly potentiated when in mixture with asiatic acid (Masoko et al., 2008), a constituent also isolated from S. densiflora leaves (Gallo et al., 2006b). O'May and Tufenkji (2011) showed that ellagitannins can block P. aeruginosa swarming motility by disturbing its quorum-sensing and biosurfactant production that are important requisites for the formation of biofilms and antibiotic resistance. Unfortunately, the similar tannins isolated from S. densiflora (Gallo et al., 2006b) could not be tested due to their small available amount and/or instability when in contact with air, yielding a thin insoluble pale yellow layer on the flask surface. Comparing literature data with the obtained results, it seems that the ellagitannins present in S. densiflora may be responsible for the high activity of HA extracts along with other constituent compounds that present antioxidant properties, like the flavonoids, so acting synergically, which might explain the MIC values of HA extracts against the bacteria in contrast to the ineffectiveness of the tannins that were tested. This fact suggests the employment of HA extracts from S. densiflora, rather than a single compound, against the A. sexdens symbiotic bacteria in order to control that common agriculture pest and also draw attention to this plant as a promising source of antibiotics.
| Tested Sample | Inhibition zone diameter of microorganisms (mm) | ||||||||
| Tc | Bc | Ec | Pa | Mr | Sa | Ca | Cl | Sc | |
| SD-HASa | 8 | 9 | 9 | 8 | 9 | 13 | 0 | 0 | 0 |
| SD-HARa | 10 | 9 | 9 | 8 | 10 | 11 | 0 | 0 | 0 |
| SD-HALa | 9 | 11 | 11 | 11 | 11 | 11 | 0 | 0 | 0 |
| SD-HATa | 7 | 9 | 9 | 9 | 10 | 14 | 0 | 0 | 0 |
| Tetracyclinb | - | 22 | 24 | 10 | 12 | 22 | - | - | - |
| Nystatinb | 32 | - | - | - | - | - | 20 | 24 | 25 |
| aHydroalcoholic extracts: SD-HAS: stem, SD-HAR: root bark, SD-HAL: leaves, SD-HAT: twigs. Test concentration: 1000 µg/disk. bControl concentration: tetracyclin = 20 µg/disk (positive control for tested bacteria: Bc, Bacillus cereus; Ec, Escherichia coli; Pa, Pseudomonas aeruginosa; Mr, Micrococcus roseus; Sa, Staphylococcus aureus); nystatin = 20 µg/disk (positive control for tested fungus and yeasts: Ca, Candida albicans; Cl, Cryptococcus laurentii; Sc, Saccharomyces cerevisiae; Tc, Trichosporon cutaneum) |
|||||||||
| Tested Sample | Antibacterial activity MIC (µg/mL) | ||||
| Bc | Ec | Pa | Mr | Sa | |
| SD-HASa | >1000 | >1000 | <31.3 | >31.3 | >250 |
| SD-HARa | >1000 | >1000 | <31.3 | >31.3 | >250 |
| SD-HALa | >1000 | >1000 | <31.3 | >125 | >250 |
| SD-HATa | >1000 | >1000 | <31.3 | >125 | >250 |
| Tetracyclineb | 1.8 | 3.7 | 12.5 | >25 | 3.7 |
| aHydroalcoholic extracts: SD-HAS: stem, SD-HAR: root bark, SD-HAL: leaves, SD-HAT: twigs. Tested concentrations: 1000, 500, 250, 125, 62.5 and 31.3 µg/mL. bPositive control (tested concentrations ranging 25.0 to 0.01 µg/mL). Tested microorganisms: Bc, Bacillus cereus; Ec, Escherichia coli; Pa, Pseudomonas aeruginosa; Mr, Micrococcus roseus; Sa, Staphylococcus aureus. |
|||||
Pectinases inhibition assay
Since tannins can bind with proteins or react with weak alkalis or acids, yielding non-specific enzymatic inhibition effect (Chalton et al., 2002; Khambabaee and Ree, 2001), the influence of this class of substances on the reagents of the enzymatic assay was previously assessed in order to check possible chemical interactions between them. The mixture containing one of the tannins (9, 10, 11 or 12) along with either pectin or buffer did not become cloudy or precipitated after the test had been performed, which might indicate absence of chemical interactions between the tannins and those reagents and, consequently, no influence in the final results. Conversely, the mixture containing either one of the tannins and the pectinases or DNSA revealed a significant OD increase, proving the occurrence of reaction between the tested tannins and those reagents.
TABLE 3 summarizes the enzymatic inhibitory activity of HA extracts from S. densiflora and some of the isolated compounds. The SD-HAS and SD-HAR extracts as well as the compounds 9, 11, and 12 exhibited potential outcomes. In addition, it was observed a significant difference between the effects of tannins 10 and 11, indicating the importance of a gallic ester group for a better activity. On the other hand, compounds 3 and 8 caused an increase in the pectinases activity. It is known that pectinases can present glycosidase activity, which induces glycosilated compound hydrolysis yielding the respective aglycone (Versari et al., 1997). This might be the reason why compounds 3 and 8 enhanced pectinases activity rather than causing its inhibition. However, it was noteworthy that tannins 9, 11 and 12 were able to inhibit pectinases activity significantly (TABLE 3), even when the preliminary tests predicted an opposite effect. Thus, one may speculate that the total tannin amounts were not completely hydrolyzed, and that the remaining molecules were capable of causing the observed result. Consequently, a higher amount of the test compound would be required to cause the complete enzyme inhibition or the hydrolytic products might be presenting a supplementary effect. Though all these assumptions need further experiments to be corroborated, the results suggest tannin 12 has potential to be explored as inhibitor of L. gongylophorus pectinases.
| Tested Sample | P valued | Concentration µg/mL | Inhibition of pectinases activity (%) |
| SD-HASa | ≤ 0.05 | 670 | 27* |
| SD-HATa | > 0.05 | 670 | 0 |
| SD-HARa | ≤ 0.001 | 670 | 29* |
| SD-HALa | > 0.05 | 670 | 0 |
| 1b | > 0.05 | 200 | 5 |
| 3b | ≤ 0.05 | 200 | 8** |
| 4b | > 0.05 | 200 | 4 |
| 5c | ≤ 0.05 | 200 | 0 |
| 6c | > 0.05 | 200 | 0 |
| 7c | > 0.05 | 200 | 3 |
| 8c | ≤ 0.05 | 200 | 5** |
| 9c | ≤ 0.001 | 200 | 60* |
| 10c | ≤ 0.001 | 200 | 4 |
| 11c | ≤ 0.001 | 200 | 28* |
| 12c | ≤ 0.05 | 200 | 15* |
| aHydroalcoholic extracts of S. densiflora: SD-HAS: stem, SD-HAR: root bark, SD-HAL: leaves, SD-HAT: twigs. bCompound isolated from S. densiflora. cCompound isolated from V. polygama. dP values ≤ 0.05 were considered significantly different from control. *Significant enzymatic inhibitory activity in relation to the control. **Increase of enzymatic activity in relation to the control. |
|||
L. gongylophorus growth inhibition
In general, the HA extracts from S. densiflora assayed against L. gongylophorus growth showed none or just moderate activity (TABLE 4), as observed in SD-HAS effect (20% inhibition). After several chromatographic separation steps, SD-HAS yielded the tannins 9 and 10. Compound 10 displayed no effect when tested at the concentration of 50 µg/mL (data not shown), but exhibited the same activity of the ori ginal extract at 100 µg/mL (TABLE 4). Compound 9 was not tested due to its insufficient amount. Compounds 5, 7 and 11 revealed a lack of activity. However, it is interesting to notice that either syringaldehyde, which is structurally different from syringic acid (7) just by a functional aldehyde group, or vanillic acid, which lacks a hydroxy group but bears an extra methyl group in comparison with gallic acid (5), caused 80% of fungal growth inhibition at the same tested concentration (Souza et al., 2005). The glycosilated flavonoid 4 and triterpene 8 displayed a weak effect on the fungal development, whereas compounds 1, 2 and 6 were twice more active, indicating that an additional sugar molecule may reduce the fungal growth inhibitory activity. Djoukeng and coworkers (2005) observed analogous trend assessing glycosilated triterpenes and their respective aglycones against some bacteria. On the other hand, the glycosylated phenolic acid 3 and tannin 12 caused 60 and 80% of inhibition on L. gongylophorus growth, respectively. Ellagic acid glycosides related to compound 12 also displayed significant activity against Magnaporthe grisea spore development (Zhou et al., 2007), which points out those compounds as promising lead antifungal substances.
| Extract or or compound | Inhibition on fungus growth (%)d |
| SD-HASa | 20 |
| SD-HATa | 0 |
| SD-HARa | 0 |
| SD-HALa | 0 |
| 1b | 40 |
| 2b | 40 |
| 3b | 60 |
| 4b | 20 |
| 5c | 0 |
| 6c | 40 |
| 7c | 0 |
| 8c | 20 |
| 10c | 20 |
| 11c | 0 |
| 12c | 80 |
| aHydroalcoholic extracts of S. densiflora: SD-HAS: stem, SD-HAR: root bark, SD-HAL: leaves, SD-HAT: twigs. Tested concentration: 1000 µg/mL. bCompound isolated from V. polygama. Tested concentration: 50 µg/ mL. cCompound isolated from S. densiflora. Tested concentration: 50 µg/ mL, except for compounds 10 and 12 (100 µg/mL). dControl with or without dilution solvent: 0% inhibition; the dry weight of the fungal suspension presented a mean of 7.2 mg/mL. |
|
Conclusion
The results obtained with the antimicrobial, growth inhibition and enzymatic inhibition assays point out the use of HA extracts of S. densiflora as a promising alternative method in the control of A. sexdens rubropilosa in the field. Also, the results showed HA extracts of S. densiflora as a prospective source of antibiotics.
Acknowledgements
The authors are grateful to Dr. Fátima Regina Salimena-Pires (UFJF) for botanical identification of Vitex, to ALCOA Alumínio S/A for facilitating the plant collections, and to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for finacial support. The authors declare that there is no conflict of interest in this research.
References
Agrawal, P.K. (ed.). 1989. Carbon-13 NMR of Flavonoids. Elsevier, Amsterdam, Netherlands.
Bauer, A.W.; Kirby, W.M.M.; Sherris, J.C.; Turck, M. 1966. Antibiotic susceptibility testing by a standardized single disk method. American Journal of Clinical Pathology, v 45, n.4, p. 493-496.
Bicalho, K.U.; Terezan, A.P.; Martins, D.C.; Freitas, T.G.; Fernandes, J.B.; Silva, M.F.G.F.; Vieira, P.C.; Pagnocca, F.C.; Bueno, O.C. 2012. Evaluation of the toxicity of Virola sebifera crude extracts, fractions and isolated compounds on the nest of leaf-cutting ants, Psyche: A Journal of Entomology, article ID 785424.
Bigi, M.F.A.M.; Torkomian, V.L.V.; Groote, S.T.C.S.; Hebling, M.J.A.; Bueno, O.C.; Pagnocca, F.C.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.G.F. 2004. Activity of Ricinus communis (Euphorbiaceae) and ricinine against the leaf-cutting ant Atta sexdens rubropilosa (Hymenoptera: Formicidae) and the symbiotic fungus Leucoagaricus gongylophorus. Pest Management Science, v 60, n.9, p. 933-938.
Bisoli, E.; Garcez, W.S.; Hamerski, L.; Tieppo, C.; Garcez, F.R. 2008. Bioactive pentacyclic triterpenes from the stems of Combretum laxum, Molecules, v 13, n.11, p. 2717-2728.
Brunskole, M.; Zorko, K.; Kerbler, V.; Martens, S.; Stojan, J.; Gobec, S.; Rizner, T.L. 2009. Trihydroxynaphthalene reductase of Curvularia lunata - A target for flavonoid action. Chemico-Biological Interactions, v 178, n. 1-3, p. 259-267.
Buzzini, P.; Arapitsas, P.; Goretti, M.; Branda, E.; Turchetti, B.; Pinelli, P.; leri, F.; Romani, A. 2008. Antimicrobial and antiviral activity of hydrolysable tannins. Mini-Reviews in Medicinal Chemistry, v 8, n.12, p. 1179-1187.
Carreiro, S.C.; Pagnocca, F.C.; Bueno O.C.; Bacci Jr., M.; Hebling, M.J.A.; Silva, A.O. 1997. Yeasts associated with nests of the leaf-cutting ant Atta sexdens rubropilosa Forel, 1908. Antonie van Leeuwenhoek, v 71, n.3, p. 243-248.
Cazal, C.M.; Domingues, V.C.; Batalhão, J. R.; Bueno, O.C.; Rodrigues Filho, E.; Silva, M.F.G.F.; Vieira, P.C.; Fernandes, J.B. 2009. Isolation of xanthyletin, an inhibitor of ants symbiotic fungus, by high-speed-counter-current chromatography. Journal of Chromatography A, v 1216, n.19, p. 4307-4312.
Chalton, A.J.; Baxter, N.J.; Khan, M.L.; Moir, A.J.G.; Haslam, E.; Davies, A.P.; Williamson, M.P 2002. Polyphenol/peptide binding and precipitation. Journal of Agricultural and Food Chemistry, v. 50, n.6, p. 1593-1601.
Dayrit, F.M.; Lapid, M.R.G.; Cagampang, J.V.; Lagurin, L.G. 1987. Phytochemical studies on the leaves of Vitex negundo, L. (“Lagundi”) I. Investigation of the bronchial relaxing constituents,” Philippine Journal of Science, v. 116, n.4, p. 403-410.
Djoukeng, J.D.; Abou-Mansour, E.; Tabacchi, R.; Tapondjou, A.L.; Boudab, H.; Lontsi, D. 2005. Antibacterial triterpenes from Syzygium guineense (Myrtaceae). Journal of Ethnopharmacology, v 101, n.1, p. 283-286.
Du, Y.G.; Chu, H.; Wang, M.F.; Chu, I.K.; Lo, C. 2010. Identification of flavone phytoalexins and a pathogen-inducible flavone synthase II gene (SbFNSII) in sorghum. Journal of Experimental Botany, v 61, n.4, 983-994.
Erthal Jr., M.; Silva, C.P.; Cooper, R.M.; Samuels, R.J. 2009. Hydrolytic enzymes of leaf-cutting ant fungi. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, v 152, n.1, p. 54-59.
Fogliani, B.; Raharivelomanana, P.; Bianchini, J.P.; Bouraima-Madjébi, S.; Hnawia, E. 2005. Bioactive ellagitannins from Cunonia macrophylla, an endemic Cunonaceae from New Caledonia. Phytochemistry, v 66, n.2, p. 241-247.
Gallo, M.B.C.; Marques, A.S.F.; Vieira, P.C.; Silva, M.F.G.F.; Fernandes, J.B.; Silva, M.; Guido, R.V.; Oliva, G.; Thiemann, O.H.; Albuquerque, S.; Fairlamb, A.H. 2008. Enzymatic inhibitory activity and trypanocidal effects of extracts and compounds from Siphoneugena densiflora O. Berg and Vitex polygama Cham. Zeitscrift für Naturforschung, v. 63c, n.(5-6), p. 371-382.
Gallo, M.B.C.; Rocha, W.C.; Cunha U.S.; Diogo, F.A; Silva, F.C.; Vieira, RC.; Vendramin, J.D.; Fernandes, J.B.; Silva, M.F.G.F. ;Batista-Pereira, L.G. 2006a. Bioactivity of extracts and isolated compounds from Vitex polygama (Verbenaceae) and Siphoneugena densiflora (Myrtaceae) against Spodoptera frugiperda (Lepidoptera: Noctuidae). Pest Management Science, v 62, n.11, p. 1072-1081.
Gallo, M.B.C.; Silva, F.C.; Vieira, P.C.; Fernandes, J.B.; Silva, M.F.G.F. 2006b. New natural products from Siphoneugena densiflora Berg (Myrtaceae) and their chemotaxonomic significance,” Journal of the Brazilian Chemical Society, v. 17, n.2, p. 279-288.
Harborne, J.B.; Williams, C.A. 2000. Advances in flavonoid research since 1992. Phytochemistry, v. 55, n.6, p. 481-504.
Hung, C.; Yen, G. 2002. Antioxidant activity of phenolic compounds isolated from Mesona procumbens Hemsl. Journal of Agricultural and Food Chemistry, v 50, n.10, p. 2993-2997.
Khambabaee, K.; Ree, T.V. 2001. Tannins classification and definition. Natural Products Reports, v. 18, n.6, p. 641-649.
Li, X.; Joshi, A.S.; Elsohly, H.N.; Khan, S.I.; Jacob, M.R.; Zhang, Z.; Khan, I.A.; Ferreira, D.; Walker, L.A.; Broedel Jr., S.E.; Rauli, R.F.; Cihlar, R.L. 2002. Fatty acid synthase inhibitors from plants: isolation, structure elucidation and SAR studies. Journal of Natural Products, v 65, n.12, p. 1909-1914.
Mahato, S. B.; Kundu, A. P. 1994. 13C NMR spectra of pentacyclic triterpenoids - a compilation and some salient features, Phytochemistry, v 37, n.6, p. 1517-1575.
Martin, M.M.; Weber, N.A. 1969. Cellulose-utilizing capability of fungus cultured by the Attini ant Atta colombica tonsipes. Annais of the American Entomology Society, v 62, n.6, p. 1386-1387.
Martin, M.M.; Boyd, N.D.; Gieselmann M.J.; Silver, R.G.J. 1975. Activity of faecal fluid of a leaf-cutting ant toward plant-cell wall polysaccharides. Journal of Insect Physiology, v 21, n.12, p. 1887-1892.
Masoko, P.; Mdee, L.K.; Mampuru, L.J.; Eloff, J.N. 2008. Biological activity of two related triterpenes isolated from Combretum nelsonii (Combretaceae) leaves. Natural Products Research, v 22, n.12, p. 1074-1084.
Miller, G.L. 1959. Use of dinitrosalycilic acid reagent for determination of reducing sugar. Analytical Chemistry, v 31, n 3, p. 426-429.
O’May, C.; Tufenkji, N. 2011. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Applied and Environmental Microbiology, v. 77, n.9, p. 3061-3067.
Okuda, T; Yoshida, T.; Ashida, M.; Yazaki, K. 1983. Tannins of Casuarina and Stachyurus species. Part I. Structures of pedunculagin, casuarictin, strictinin, casuarinin, casuariin, and stachyurin. Journal of the American Chemical Society Perkin Transactions I, p. 1765-1772.
Pagnocca, F.C.; Silva, O.A.; Hebling-Beraldo, M. J. ; Bueno, O.C.; Fernandes, J.B.; Vieira, P.C. 1990. Toxicity of sesame extracts to the symbiotic fungus of leaf-cutting ants. Bulletin of Entomology Research, v 80, n.3, p. 349-352.
Salvat, A.; Antonacci, L.; Fortunato, R.H.; Suarez, E.Y; Godoy, H.M. 2001. Screening of some plants from Northern Argentina for their antimicrobial activity. Letters of Applied Microbiology, v 32, n.5, p. 293-297.
Santos, A.V.; Dillon, R.J.; Dillon, V.M.; Reynolds, S.E.; Samuels, R.I. 2004. Occurrence of the antibiotic producing bacterium Burkholderia sp. in colonies of the leaf-cutting ant Atta sexdens rubropilosa. FEMS Microbiology Letters, v 239, n.2, p. 319-323.
Schinor, E.C.; Salvador, M. J.; Ito, I.Y.; Dias, D.A. 2007. Evaluation of the antimicrobial activity of crude extracts and isolated constituents from Chresta scapigera. Brazilian Journal of Microbiology, v 38, n.2, p. 145-149.
Serrano, J.; Puuponen-Pimia, R.; Dauer, A.; Aura, A.M.; Saura-Calixto, F. 2009. Tannins: current knowledge of food sources, intake, bioavailability and biological effects, Molecular Nutrition & Food Research, v 53, suppl.2, p. S310-S329.
Shimomura, H.; Sashida, Y; Adachi, T. 1988. Phenylpropanoid glycose esters from Prunus buergeriana. Phytochemistry, v 27, n.2, p. 641-644.
Silva, A.; Bacci Jr., M.; Pagnocca, F.C.; Bueno, O.C.; Hebling, M.J.A. 2006. Production of polysaccharidases in different carbon sources by Leucoagaricus gongylophorus Möller (Singer), the symbiotic fungus of the leaf-cutting ant Atta sexdens Linnaeus. Current Microbiology, v 53, n.1, p. 68-71.
Snedecor, G.W.; Cochran, W.G. 1989. Statistical Methods, 8th ed. Iowa State University Press, Ames, USA.
Souza, R.C.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.G.F.; Godoy, M.F.P.; Pagnocca, F.C.; Bueno, O.C.; Hebling, M.J.A.; Pirani, J.R. 2005. A new imidazole alkaloid and other constituents from Pilocarpus grandiflorus and their antifungal activity. Zeitscrift für Naturforschung, v 60b, n.7, p. 787-791.
Taguri, T.; Tanaka, T.; Kouno, I. 2004. Antimicrobial activity of 10 different plant polyphenols against bacteria causing food-borne disease. Biological and Pharmaceutical Bulletin, v. 27, n.12, p. 1965-1969.
Tiang, G.; Zhang, T.; Yang, F.; Ito, Y 2000. Separation of gallic acid from Cornus officinallis Sieb. et Zucc by high-speed counter-current chromatography. Journal of Chromatography A, v. 886, n.1, p. 309-312.
Versari, A.; Biesenbruch, S.; Barbant, D.; farnell, P.J.; Galassi, S. 1997. Effects of pectolytic enzymes on selected phenolic compounds in strawberry and raspberry juices. Food Research International, v. 30, n.7, p. 811-817.
Wang, X.G.; Wei, X.Y; Tiang, Y.Q.; Sheng, L.T.; Xu, H.H. 2010. Antifungal flavonoids from Ficus sarmentosa var. henryi (King) Corner. Agricultural Sciences in China, v 9, n.5, p. 690-694.
Weber, N.A. 1972. Gardening Ants: the Attines, v.92. The American Philosophical Society, Philadelphia, USA.
Yanamaka, F.; Hatano, T.; Ito, H.; Taniguchi, S.; Takahashi, E.; Okamoto, K. 2008. Antibacterial effects of guava tannins and related polyphenols on Vibrio and Aeromonas species. Natural Products Communications, v 3, n.5, p. 711-720.
Zhou, L.G.; Li, D.; Jiang, W.B.; Qin, Z.Z.; Zhao, S.; Qiu, M.H.; Wu, J.Y 2007. Two ellagic acid glycosides from Gleditsia sinensis Lam. with antifungal activity on Magnaporthe grisea. Natural Product Research, v 21, n.4, p. 303-309.