Artigo de Pesquisa
Emetine and cephaeline production and regulation by in vitro propagation of Psychotria ipecacuanha (Brot.) Stokes in semi-solid media and temporary immersion bioreactor
Abstract
Psychotria ipecacuanha, is a plant species with known medicinal properties that is critically endangered due to overexploitation of natural populations. Although the difficulties in conventional propagation by seed and by vegetative propagation are generally understood, the present study enhances our knowledge by describing efficient plant regeneration and root induction protocols for P. ipecacuanha while comparing alkaloid content (emetine and cephaeline) in in vitro-derived tissues. Stem node explants were cultured on MS medium MS supplemented with indolbutiric acid (IBA) in semi-solid media and the RITA® temporary immersion bioreactor. The highest root formation (81%) was in MS + 1.5 mg L−1 IBA in the bioreactor. After 24 months of acclimatization, the plants cultivated in MS + 0.50 and 1.0 mg L−1 of IBA had the highest number of roots (3), with mean values of 10.47 and 9.40 cm, respectively. The cultures coming from 1.0 mg L−1 and 0.5 mg L−1 IBA in the bioreactor contained higher cephaeline content, with a relative area of 14.2 and 14.9%, respectively. For emetine, the 1.0 mg L−1 IBA cultures in the bioreactor, 0.5 mg L−1 IBA and MS0 cultures contained higher content than the other treatments, with a relative area of 10.2, 10.2 and 10.1%, respectively.
- Keywords:
- Plant biotechnology.
- Tissue culture.
- Ipecac.
- Temporary immersion.
- Secondary metabolites.
Introduction
Psychotria ipecacuanha Brot. Stokes (Rubiaceae), commonly known as ipeca or poaia, is recognized worldwide as one of the most important medicinal plant species in the pharmaceutical industry due to the presence of bioactive alkaloids in the roots, primarily emetine, cephaeline and psychotrine[1,2]. Alkaloids have emetic action in bronchial diseases (e.g., anti-inflammatory properties) and can combat both fever and malaria, and also act as inhibitors of protein and DNA synthesis[3,4]. In addition, the root extract of this species is used and recognized in the Pharmacopoeia as an amebicide and expectorant[5-8].
Several substances are found in poaia roots: starch, reducing sugars, resins, tannins, malic acid, citric, ipecacuanic, and the alkaloids that justify the plant therapeutic properties: emetine, cephaeline, psychotrine, emetamine o-methyl psicotrin, and proto-emetim. The alkaloids correspond to 2 to 3% of the root dry weight and are mainly located in the cortical parenchyma (2.5%) and in small quantities in the woody zone [9].
Psychotria ipecacuanha occurs in highly shaded underbrush areas in Central America (Nicaragua, Costa Rica and Panama) and northern South America (Colombia), the southwestern part of the Brazilian Amazon forest (Rondônia and Mato Grosso States) and the Brazilian Atlantic forest, especially in the States of Minas Gerais, Espírito Santo, Rio de Janeiro, and Bahia[1,6,10,11]. Brazilian ipecac is the preferred species in the world market because it has a higher concentration of emetic alkaloids, which has led Brazil to become one of the main exporters of this product[12].
International demand for ipecac leads to a profitable crop, yet the Brazilian producer (collector/intermediary) receives only USD $ 24.31/kg root tissue (Ethnobotanical Gardener, 2016). This species is threatened by genetic erosion or extinction in Brazil because it has undergone an intense extraction process in the past two centuries, expanding agricultural frontiers with consequent reduction of habitat[13].
Seed propagation in P. ipecacuanha easily lose viability after collection, have a low rate of germination, and show slow growth and premature death of plants under natural conditions[14-17]. Vegetative propagation is limited by the slow growth of plants. Consequently, there is an urgent need for the development of a protocol for large-scale propagation of selected material aimed at the reintroduction into the habitat as well as the production of material with a higher alkaloid content, mainly for the establishment of the best crop yield for supplying pharmaceutical industries.
In vitro culture techniques are presented as an alternative for the mass propagation and conservation of endangered, rare or endemic species[18]. Among other advantages, these techniques improve upon conventional propagation due to the generation of seedlings with apical meristem and root simultaneously (i.e., so that a later phase of rooting is not needed) in addition to ease of scheduling and less probability of genetic variation[18-20]. Despite these advantages, this process should be "personalized" considering the differential responses of each genotype, growth regulators, explant type, donor plant age, and growth conditions, among other factors[18].
Biotechnology provides the opportunity through in vitro culture to use cells, tissues, organs, or even the entire organism by genetically manipulating them to obtain the desired substances[21,22]. In some cases, the production of biomolecules has been achieved via cell culture on an industrial scale, such as shiconin, berberine and taxol, or for biomass as in the case of ginseng roots. However, for many of the drugs of interest, production is rare to absent in plant cell culture, typically because production is controlled in specific ways in the tissues. In this way, the lack of differentiation would result in the loss of productive capacity; thus, many studies currently aim to optimize the means of growth and production or to select more productive cell lines. In addition, other approaches such as the growth of differentiated cells (root and shoot culture) and the induction of biosynthetic pathways using stimulators are strategies that have shown excellent results within plant tissue culture[23].
The use of natural products as a model for drug synthesis or simple use by the population is a growing trend, and many studies have evaluated these products. In relation to P. ipecacuanha, most studies have addressed chemical and pharmacological properties[9].
Literature review reveals a high medicinal and commercial value of Psychotria ipecacuanha roots, but there is a strong variability in the production of the chemical compounds of interest (emetine and cephaeline) and little information on the standardization of treatment of raw plant material to increase these plant constituents. The aim of this study was to develop a protocol to produce in vitro seedlings and roots of P. ipecacuanha with subsequent chemical characterization in order to obtain standardized extracts. These results will help to stimulate the commercial cultivation of this species with selected seedlings and higher productivity of the pharmacological compounds of interest.
Materials and Methods
Plant material
In vitro cultures acquired from the germplasm bank of the Embrapa Amazônia Oriental (identified by the IAN Herbarium - Embrapa Amazônia Oriental) were used to obtain the plant material for the establishment of micropropagation and root culturing protocols of Psychotria ipecacuanha, which were used as donors of explants for the tests with different concentrations of the growth regulator Indolbutiric Acid (IBA)[24,25].
Treatment with growth regulator in semi-solid culture media in the temporary immersion bioreactor
Regenerated plantlets from nodal segments were tested in culture media according Murashige and Skoog, 1962 (MS) with different concentrations (0.5; 1.0; 1.5 and 2.0 mg L−1) of the growth regulator Indolbutiric Acid (IBA) and the control (MS0) in semi-solid media and RITA® (Vitropic S/A) temporary immersion bioreactors (n=30 per treatment) to evaluate root production[26].
Glass vials containing 40 mL of semi-solid medium and RITA® (Vitropic S/A) temporary immersion bioreactors containing 250 ml of liquid medium (n=30 per treatment) were used. The immersion frequency used for the bioreactor system was ten seconds every three hours. The cultures were maintained under white light illumination (Sylvania fluorescent tubes) under 1.6 W m-2, 30 μmol m-2 s-1 with a daily photoperiod of 16 hours at 25 ± 1 °C, for 10 days. After this period, the explants were transferred to flasks containing 40 mL of MS medium free of growth regulators (MS0) and kept in the growth room.
After 60 days, the following parameters were evaluated: rooting percentage, average number of roots per plant, and mean root compliance.
Statistical analysis
The effect of different concentrations of the growth regulator IBA on root development per explant was evaluated by analysis of variance (ANOVA) and the means were compared by a Tukey-Kramer test at a significance level of 5%. These analyses were performed using Graph Pad in Stat version 3.01. In the analysis of the rooting percentages according to the medium used, we ran a percentage difference test (p1 and p2) at 5% significance level using Statistica for WindowsTM, 5.0 version.
We used a completely randomized 5 x 6 factorial experimental design consisting of two types of containers (250 mL flasks and the RITA® bioreactor) with three replicates per treatment and 30 explants per replicate, which were evaluated at 60 days after transfer to the MS0 medium.
Acclimatization
Plantlets grown in all in vitro treatments were transferred to polypropylene bags (1 kg) containing substrate and remained for 30 days in a greenhouse with a fogging system. At the end of this period, the plants were transferred to another greenhouse with a micro sprinkler system where they remained for another 23 months. At the end of this period, the plants were used as root donors for the phytochemical tests.
Alkaloid content analysis
The roots of acclimatized plants (500 mg) were air dried for a period of 5 days and submitted to extraction. Each root sub-sample (500 mg) was vortexed for one minute with a mixture of 10% ammonium hydroxide (NH4OH) (5 mL) and ethyl ether ((C2H5)2O) (5 mL) in a glass container. The organic phase was transferred to another glass vessel and vortexed for one minute with 10% acetic acid (CH3CO2H) (5 mL). The aqueous phase was removed and treated with NH4OH to pH 10 and then vortexed for 1 minute with ethyl ether ((C2H5)2O (5 mL).
The chromatographic method was developed for relative qualitative analysis of the fractions using high-efficiency thin layer chromatography (HPTLC) with densitometry.
The chromatoplaques (normal phase) were eluted in an 8:2 chloroform/methanol mixture and developed with UV light at 254 and 366 nm.
The relative quantification of the alkaloids (emetine and cephaeline) was based on the calculation of the spot absorption area. Using a densitometer, the density of the applied samples was analyzed comparing the different concentrations of the standard on the chromatographic plate. The absorption area of the respective retention factors (Rfs) of the alkaloids were used to calculate the percentages as follows: [Area of each sample (Rf area of emetine or cephaeline) / Total area] x 100 = % of alkaloids.
Results and Discussion
Data from development in different culture media were compared with those obtained from plantlets treated with MS0.
The addition of 1.5 mg L−1 IBA to bioreactor MS medium resulted in 81% rooting of the explants with an average yield of 3.41 roots of 0.6 cm at 60 days after transfer to MS0. The semi-solid culture medium supplemented with 1.5 mg L−1 IBA led to 20% rooting of the seedlings, with a mean root yield of 1.8 roots of 0.46 cm (TABLE 1). Regarding the use of MS0, in both treatments there were no signs of rooting.
| Culture Media | Rooting (%) | Root Number | Root Length (cm) |
| MS0 | 0 | - | - |
| MS0_bioreactor | 0 | - | - |
| 0.5 mg L−1 IBA | 13h | 1.00g | 0.25f |
| 0.5 mg L−1 IBA_bioreactor | 43b | 2.83c | 0.54c |
| 1.0 mg L−1 IBA | 36d | 2.22e | 0.47d |
| 1.0 mg L−1 IBA _bioreactor | 30e | 3.38b | 0.64a |
| 1.5 mg L−1 IBA | 20g | 1.80f | 0.46d |
| 1.5 mg L−1 IBA_bioreactor | 81a | 3.41b | 0.60b |
| 2.0 mg L−1 IBA | 40c | 2.50d | 0.31e |
| 2.0 mg L−1 IBA_bioreactor | 23f | 4.00a | 0.57b |
| *Values in the same column followed by the same letter do not differ statistically from each other (significance level 5%). | |||
Bioreactors may serve as an alternative for preserving viability in the micropropagation technique of several species, primarily by eliminating and/or automating some steps of plant propagation; this is because the temporary immersion system provides a highly aerobic system for plant growth through forced ventilation through the vessel lid. However, the immersion times and duration and frequency of immersion are the most decisive parameters for successful micropropagation, as they influence nutrient and water uptake and consequently, hyperhidrosis of the cultured material[27-29].
According to Smet and Beeckman[30], adventitious root initiation involves meristematic cell niche formations (initial cells and/or target cells) which are dependent on external and internal factors. The differentiation of the meristematic cell niche and the formation of new roots involves stem tissue disruptions to form connections with the conductive vascular tissues induced by auxins[31,32]. Brondani et al.[33], working with Eucalyptus benthamii x Eucalyptus dunnii, observed that the ½ MS culture medium supplemented with 1 mg L−1 IBA induced 75% rooting of micro-cuttings.
According to Souza e Pereira[34], the most common auxins used for rooting are naphthalene acetic acid (NAA), indolbutiric acid (IBA) and indolacetic acid (IAA). The main differences among them are molecular structure and the stability, where NAA is more stable than IAA; thus, most in vitro rooting protocols use NAA and IBA auxins.
Regardless of the auxin formula, excessive concentration in the culture medium can be toxic, promoting callus formation at the micro-cutting bases and compromising rooting and shoot growth. Generally, the root and shoot vascular concentration is very fragile and callus formation can compromise adventitious root functionality[31]. However, callus formation was not observed on explants of Psychotria ipecacuanha. Based on these results, the treatment in the bioreactor in MS media with 1.5 mg L−1 IBA is considered the most suitable for in vitro rooting of Psychotria ipecacuanha, resulting in 81% rooting.
The plantlets produced in all test treatments can be acclimatized from 60 days of cultivation, since 100% survival was obtained after 24 months of acclimatization (FIGURE 1).

| Culture Media | Rooting (%) | Roots Number | Roots Lenght (cm) |
| MS0 | 100 | 2.33c | 12.29a |
| MS0_bioreactor | 100 | 3.00a | 10.47c |
| 0.5 mg L−1 IBA | 100 | 3.00a | 9.40d |
| 0.5 mg L−1 IBA_bioreactor | 100 | 2,33c | 11.70ab |
| 1.0 mg L−1 IBA | 100 | 2.33c | 9.96cd |
| 1.0 mg L−1 IBA_bioreactor | 100 | 2,66b | 9.83d |
| 1.5 mg L−1 IBA | 100 | 1.66d | 11.13b |
| 1.5 mg L−1 IBA_bioreactor | 100 | 2.33c | 7.96f |
| 2.0 mg L−1 IBA | 100 | 2.66b | 8.58e |
| 2.0 mg L−1 IBA_bioreactor | 100 | 2.33c | 9.76d |
| *Values in the same column followed by the same letter do not differ statistically from each other (significance level 5%). | |||
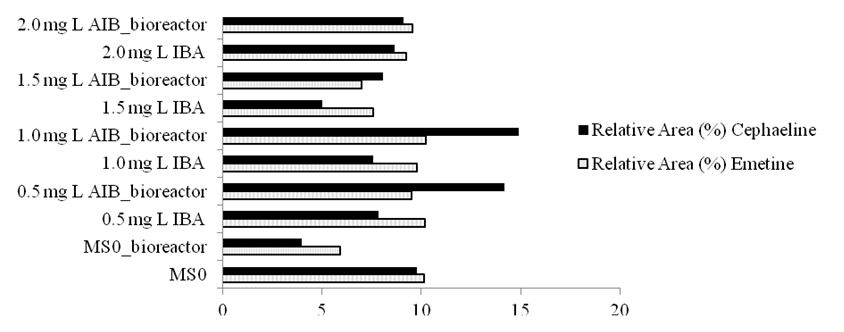
According to HPTLC alkaloid fraction analyses, there is a direct dependence on the composition and content of growth regulators in culture media, and the system used (TABLE 3 and FIGURE 2). The principal components of alkaloids of P. ipecacuanha rootsare presented in TABLE 2: Emetine (10.12; 5.93; 10.18; 9.49; 9.76; 10.23; 7.56; 6.97; 9.23 and 9.57%) and cephaeline (9.79; 3.99; 7.86; 14.17; 7.58; 14.91; 5.04; 8.07; 8.67 and 9.12) in plants cultured on MS0 media, and MS + 0.5, 1.0, 1.5, and 2.0 mg L−1 IBA in semisolid and bioreactor conditions, respectively. Comparative analysis of the HPTLC profiles showed high percentage of emetine and cephaeline in the MS+1.0 mg L−1 IBA treatment in the bioreactor, compared to other treatments.
| Culture Media | Relative Area (%) | |
| Emetine | Cephaeline | |
| MS0 | 10.12a | 9.79b |
| MS0_bioreactor | 5.93e | 3.99g |
| 0.5 mg L−1 IBA | 10.18a | 7.86e |
| 0.5 mg L−1 IBA_bioreactor | 9.49b | 14.17a |
| 1.0 mg L−1 IBA | 9.76ab | 7.58e |
| 1.0 mg L−1 IBA bioreactor | 10.23a | 14.91a |
| 1.5 mg L−1 IBA | 7.56c | 5.04f |
| 1.5 mg L−1 IBA_bioreactor | 6.97d | 8.07e |
| 2.0 mg L−1 IBA | 9.23b | 8.67d |
| 2.0 mg L−1 IBA_bioreactor | 9.57b | 9.12c |
| *Values in the same column followed by the same letter do not differ statistically from each other (significance level 5%). | ||
Due to growth conditions used in this work, the light levels, temperature, mineral composition of growth regulators, pH, and age of the explants was completely controlled.
The results show that the addition of MS+1.0 mg L−1 IBA in the culture medium in the bioreactor induced the increase of these alkaloids, but when the concentration of this growth regulator was increased the alkaloids decreased.
Root growth and the composition of the root alkaloids of P. ipecacuanha can be altered by addition of selected phytohormones to the nutrition medium.
The effects of auxin on gene activity, especially in the epidermis, explains the quantitative variation promoted by addition of auxin[35]. Auxins may affect the types of proteins formed in a plant cell before or as soon as growth promotion starts, which may explain the changes in the level of some substances through the modification of cell enzymatic patterns[36].
The effect of auxin on the levels of secondary metabolites has been previously observed. Auxin is able to stimulate the synthesis of some essential oil compounds and indole alkaloids[36,37].
The results from the present study suggest that the growth regulator IBA influences P. ipecacuanha alkaloid composition. This is an interesting result since emetine and cephaeline have great commercial value and are mainly utilized in the phytopharmaceutical industry.
Conclusion
The use of MS0 in temporary immersion bioreactor and semisolid MS medium supplemented with 0.5 mg L−1 of indolebutyric acid favored greater root production by providing 3 roots each of Psychotria ipecacuanha seedlings. However, for increased emetine and cephaeline production, the treatment producing the best results was supplementation of MS medium with 1.0 mg L−1 IBA in the temporary immersion bioreactor, with a production of 10.23 and 14.91%, respectively. Although this treatment promoted only 30% in vitro rooting of the seedlings, 100% survival was observed in the acclimatization process of all seedlings tested regardless of in vitro rooting, with no detectable morphological anomalies or variation. We therefore conclude that there is no dependence of rooting in the in vitro phase for acclimatization success.
The micropropagation protocol established here was adequate for P. ipecacuanha seedling production because it uses low concentrations of IBA (1.0 mg L−1) and results in seedlings with higher production of emetine and cephaeline, with an average yield of 10.23 and 14.91%, respectively, at 24 months after acclimatization.
Acknowledgements
We are grateful for the financial support from the Superintendência da Zona Franca de Manaus (SUFRAMA), the Instituto Nacional de Metrologia, Qualidade e Tecnologia (INMETRO), the Universidade Federal do Amazonas (UFAM) and the Empresa Brasileira de Pesquisa Agropecuária (Embrapa Amazônia Ocidental).
Referências
1. Assis, MC, Giulietti, AM. Diferenciação morfológica e anatômica em populações de "ipecacuanha" - Psychotria ipecacuanha (Brot.) Stokes (Rubiaceae). Braz J Botany. 1999; 22(2): 205-216. ISSN 1806-9959. [CrossRef].
2. Rossi AAB, Oliveira LO, Venturini BA, Silva RS. Genetic diversity and geographic differentiation of disjunct Atlantic and Amazonian populations of Psychotria ipecacuanha (Rubiaceae). Genetica - An Inter J Genetics Evol. 2009; 13: 57-67. ISSN 1573-6857. [CrossRef].
3. Agra MF, Silva KN, Basílio IJLD, França PF, Barbosa-Filho JM. Survey of medicinal plants used in the region Northeast of Brazil. Rev Bras Farmacog. July/Sept. 2008; 18: 472-508. ISSN 1981-528X. [CrossRef].
4. Burhans WC, Vassiley LT, Wu JM, Nallaseth FS, Depamphilis ML. Emetine allows identification of origins of mammalian DNA replication by imbalanced DNA synthesis, not through conservative nucleosome segregation. EMBO J. 1991; 10: 4351-4360. [CrossRef].
5. Akinboye E, Bakare O. Biological activities of emetine. The Open Nat Prod J. 2011; 4: 8-15. ISSN 1874-8481. [Link] [CrossRef].
6. Nomura T, Kutchan T. Three new o-methyltransferases are sufficient for all o-methylation reactions of ipecac alkaloid biosynthesis in root culture of Psychotria ipecacuanha. The J Biol Chem. 2010; 285 (10): 7722–7738. [CrossRef].
7. Brandão M, Zanetti N, Oliveira A, Grael C, Santos A, MonteMór R. Brazilian medicinal plants described by 19th century European naturalists and in the Official Pharmacopoeia. J Ethnopharmacol. 2008; 120: 141–148. [CrossRef].
8. Ideda K, Teshim D, Aoyama T, Satake M, Shimomura K. Clonal propagation of Cephaelis ipecacuanha. Plant Cell Reports. 1988; 7: 288-291. [CrossRef].
9. Lameira OA. Cultivo da ipecacuanha [Psychotria ipecacuanha (Brot.) Stokes]. Belém: Embrapa Amazônia Oriental. Circular Técnica. 2002;28: 1-4. [Link]
10. Skorupa LA, Assis MC. Collection and conserving Ipecac (Psychotria ipecacuanha, Rubiaceae) germplasm in Brazil. Economic botany. 1998; 52 (2): 209-210. [CrossRef].
11. Alves GR, Oliveira L, Alves MM, Silva BW. Variation in emetine and cephaeline contents in roots of wild Ipecac (Psychotria ipecacuanha). Biochem System Ecol. 2005; 33: 233-243. [CrossRef].
12. Vieira LS. Manual da medicina popular. Belém: Agronomia Vozes. 1991; 247. [Link].
13. Rocha TT, Lameira OA. Avaliação do período de floração e frutificação do BAG ipecacuanha. Em: 15° Seminário de Iniciação Científica da Embrapa. Belém, PA. Anais do 15° Seminário de Iniciação Científica da Embrapa: Embrapa Amazônia Oriental, 2011. [Link].
14. Satoko I, Kaori T, Koichiro S. Gibberellic acid improved shoot multiplication in Cephaelis ipecacuanha. In vitro Cel Develop Biol Plant. 2007; 44 (3): 216-220. [CrossRef].
15. Chatterjee K, Nandi P, Ghosh C. Cultivation and utilization of ipecac In West Bengal. In: Atal CK, Kapur BM. eds. Cultivation and utilization of medicinal plants. Jammu-Tawi, India: CSIR, Regional Research Laboratory. 1982; p.295-301. 877p. [Link].
16. Rout GR, Samantaray S, Das P. In vitro somatic embryogenesis from callus cultures of Cephaelis ipecacuanha A. Richard. Sci Horticult. 2.000; 86: 71-79. [CrossRef].
17. Jha S, Jha T. Micropropagation of Cephaelis ipecacuanha Rich. Plant Cell Reports. 1989; 8: 437-439. [CrossRef].
18. Robinson P, John JBS, Balakrishnan V. Regeneration of Plants Through Somatic Embryogenesis in Emilia zeylanica C. B. Clarke a Potential Medicinal Herb. Bot Res Inter. 2009; 2 (1): 36-41. [Link].
19. Khadke S, Kuvalekar A. Direct Somatic Embryogenesis and Plant Regeneration from Leaf and Stem Explants of Nothapodytes foetida: A Critically Endangered. Plant Species. Inter J Plant Anim Environ Sci. 2013; 3 (1): 257-264. ISSN 2231-4490. [Link].
20. Aiqing J, Xueqing G, Yan Z, Hongyan Y, Guoliang W. Advances in Somatic Embryogenesis Research of Horticultural Plants. American J Plant Sci. 2011; 2: 727-732. [CrossRef].
21. Rao SR, Ravishankar GA. Plant Cell Cultures: Chemical Factories of Secondary Metabolites. Biotechnol Adv. 2002; 20: 101-153. [CrossRef].
22. Verpoorte R, Memelink J. Engineering secondary metabolite production in plants. Cur Oppin Biotechnol. 2002; 13: 181-187. [CrossRef].
23. Lourenço MV. Biotecnologia de Plantas Medicinais: Produção de Biomoléculas. Biológico. 2003; 65 (1/2): 63-65. [Link].
24. Silva S, Pinheiro EN, Assunção LM, Silva EL, Rodrigues DC, Ferreira FF, Astolfi-Filho S. In vitro propagation of Psychotria ipecacuanha (Brot.) Stokes under different concentrations of Indoleacetic Acid. Rev Fitos. 2018; 12 (3): 263-268. [CrossRef].
25. Silva S, Astolfi-Filho S. Effect of indolebutyric acid on in vitro root production of Psychotria ipecacuanha (Brot.) Stokes (Rubiaceae). Rev Fitos. 2018; 12 (3): 218-226. ISSN 2446-4775. [CrossRef].
26. Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Physiol Plantarum. 1962; 15: 473-497. [Link].
27. Oliveira ML, Xavier A, Filho RMP, Otoni WC, Teixeira JB. Efeitos do meio de cultura e da relação BAP/ANA na multiplicação in vitro de clones de Eucalyptus grandis x E. urophylla em bioreactor de imersão temporária. Rev Árvore. 2011; 35 (6): 1207-1217. [CrossRef].
28. Berthouly M, Etienne H. Temporary immersion system: a new concept for use of liquid medium in mass propagation. In: International Symposium on Liquid Systems for in vitro Mass Propagation of Plants. Norway Proceedings. Norway: Cost 843 Working Group. 2002; 1: 37-38. [Link] [CrossRef].
29. Watt MP. The status of temporary immersion system (TIS) technology for plant micropropagation. African J Biotechnol. 2012; 11 (76): 14025-14035. [CrossRef].
30. Smet I, Beeckman T. Assymmetric cell division in land plants and algae: the driving force for differentiation. Nature Rev Mol Cell Biol. 2011; 12 (3): 177-188. [CrossRef].
31. Li SW, Xue L, Xu S, Feng H, Na L. Mediators, genes and signaling in adventitious rooting. Botanycal Review. 2009; 75 (2): 230-247. ISSN 1874-9372. [CrossRef].
32. Brondani GE, Baccarin FJB, Wit Ondas HW, Stape JL, Gonçalves AN, Almeida M. Low temperature, IBA concentrations and optimal time for adventitious rooting of Eucalyptus benthamii mini-cuttings. J Forestry Res. 2012: 23 (4): 583-592. [CrossRef].
33. Brondani GE, Dutra LF, Wendling I, Grossi F, Hansel FA, Araujo MA. Micropropagation of an Eucalyptus hybrid (Eucalyptus benthamii x Eucalyptus dunnii). Acta Scient. 2011; 33 (4): 655-663. [CrossRef].
34. Souza AV, Pereira MAS. Enraizamento de plantas cultivadas in vitro. Rev Bras Plantas Med. 2007; 9 (4): 103-117. [Link].
35. Biddington NL, Thomas TH. A modified Amaranthus betacyanin bioassay for the rapid determination of cytokinins in plant extracts. Planta. 1973; 111: 183-186. [CrossRef].
36. Mérillon JM, Liu D, Huguet F, Chénieux JC, Rideau M. Effect of calcium entry blockers and calmodulin inhibitors on cytokinin enhanced alkaloid accumulation in Catharanthus roseus cell cultures [indole alkaloids, calcium uptake]. Plant Physiol Biochem. 1991; 29: 289-296. [Link].
37. Silva S, Lage CLS, Esquibel MA, Gil RASS, Sato A. In vitro propagation of Melissa officinalis L. and production of essential oil. Plant Cell Culture e Micropropag. 2006; 2(2): 53-60. [Link].