Artigo de Pesquisa
Determination of aristolochic acids I and II in Brazilian sugar cane spirit infusions "milhomem" commonly used in northeast Brazil as popular drinks
Abstract
Aristolochic acids (AA) are phytochemicals found in plants of the genus Aristolochia belonging to the family Aristolochiaceae. These compounds bear a nitrophenanthrene carboxylic acid skeleton and are reported to be carcinogenic, mutagenic, and nephrotoxic. Sugar cane spirit infusions containing Aristolochia species are commonly used in Brazil as popular drinks, in total absence of scientific information. The presence aristolochic acids was confirmed in samples collected in popular markets of the city of Aracaju, Sergipe, Brazil. The aristolochic acids quantitative estimation was made in five samples of sugar cane spirit infusions obtained from different places of that city and were performed by high-performance liquid chromatography. The samples analyzed contained aristolochic acids I and II in concentrations ranging between 1.96 and 6.10 µg/ml for AA I and 2.22 and 11.55 µg/ml for AA II. The immediate banning of such popular drinks is recommended in view of the danger to ingest aristolochic acids, botanical products containing aristolochic acids or herbal products containing plants belonging to Aristolochiaceae family.
- Keywords:
- Milhomem.
- Sugar cane spirit infusion.
- Aristolochic acid I.
- Aristolochic acid II.
- Quantitative analysis.
Introduction
Many plants have been used for centuries in Brazilian folk medicine to treat several human ailments. Different types of preparations, such as water infusions (referred to as "teas"), hydroalcoholic extracts and juices among others are usual on these therapies. "Garrafadas" are mixed medicinal plants extracts that use sugar cane spirit, a typical Brazilian alcoholic beverage called "aguardente" or "cachaça" or wine as vehicle[1]. A very popular herbal preparation referred to as "milhomem" (obtained by infusion of several Aristolochia plant species, such as A. cymbifera, A gigantea, A. birostris with sugar cane spirit) is used to treat liver and stomach disorders, snake bites, fever and ulcer for example[2-4].With about 490 species spreading around the tropical areas[5] plants of genus Aristolochia (Aristolochiaceae) are known to contain nitrophenanthrene carboxylic acid compounds known as aristolochic acids (AA).
Aristolochic acids are strongly recorded as responsible for the nephrotoxic and genotoxic effects associated with the use of some herbal preparations[6,7]. The first evidence about the nephrotoxicity of aristolochic acids I and II was published in 1963[8]. In addition, some types of cancer specially occurring in the urinary tract have also been linked to the ingestion of AA's present in these products[9,10]. Several analogs of AA are described, but aristolochic acids I and II (FIGURE 1) have particular significance for their common occurrence[11,12].
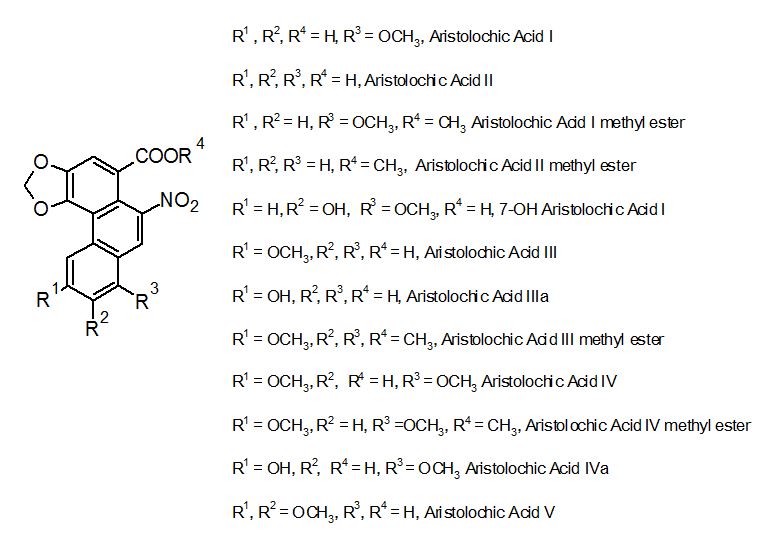
Notwithstanding the fact that FDA suggested the removal of herbal products suspected to contain aristolochic acids from the market since 2001[13], some of these products are still available for consumption[14]. Taking into consideration the risk of using products containing AA's, this work aims to evaluate the presence and levels of AA I and AA II on handmade alcoholic drinks sold in popular markets of Aracaju, capital of Sergipe, a state of the Brazilian Northeast region.
Material and Methods
General
Reagents and solvents were PA grade (Tedia Brazil) except those used for HPLC, analysis that were UV/HPLC grade (Merck). The ultrapurified water used was obtained with a Milli-Q apparatus (Millipore). Aristolochic acids I and II standards were obtained from Sigma-Aldrich. Analytical data was processed using Minitab 17 statistical software (Minitab Inc. 2006, State College, PA, USA).
Sample collection and preparation
Samples of sugar cane spirit infusions (n = 5) were collected on different places at the city of Aracaju during the year of 2017. The undiluted samples were directly filtered through PTFE syringe filters (0.22 µm pore size, Tedia Brazil) to 2 mL vials and submitted to HPLC analysis.
Quantitation
Calibrating solutions were prepared by using a standard of aristolochic acids purchased from Sigma (a mixture of 28% AA I and 66% AA II). Dilutions were made with aliquots of a stock solution (10 mg/100 mL of methanol) transferred to 50 ml volumetric flasks and added with methanol to a final volume. The solutions (made in triplicate) were submitted to HPLC analysis (see conditions below), the peak areas of AA I and AA II were integrated using the software and used to build a calibration curve (R2 = 0.999; limits of detection = 0.025 (AA I) and 0.041 (AA II) µg/mL); the limits of quantitation were determined as 0.27 (AA I) and 0.38 (AA II) µg/mL. The sample solutions (made in triplicate) were submitted to HPLC analysis (see conditions below).
HPLC analysis conditions
An HPLC Agilent 1200 series equipped with photodiode array detector was used for sample analysis. The method was as follows: column: RP18 Waters XTerra, 4.6 mm x 250 mm, 5 μm particle size; mobile phase, aqueous 0.5 % acetic acid (A) and methanol 0.5% acetic acid (B); gradient elution: 60% to 100% of B in 21 minutes; flow rate, 1.0 mL/min.; injection volume: 20 µL. UV detection, 254, 300 and 320 nm; retention times AAI: 22.6 min and AAII: 19.9 min. The retention times and UV spectra of samples and standards were compared in order to confirm identification [15].
Results and Discussion
Five samples were tested for the presence of AAI and AAII. Quantitative analysis was carried out on all samples. Typical chromatograms are displayed (FIGURE 2).
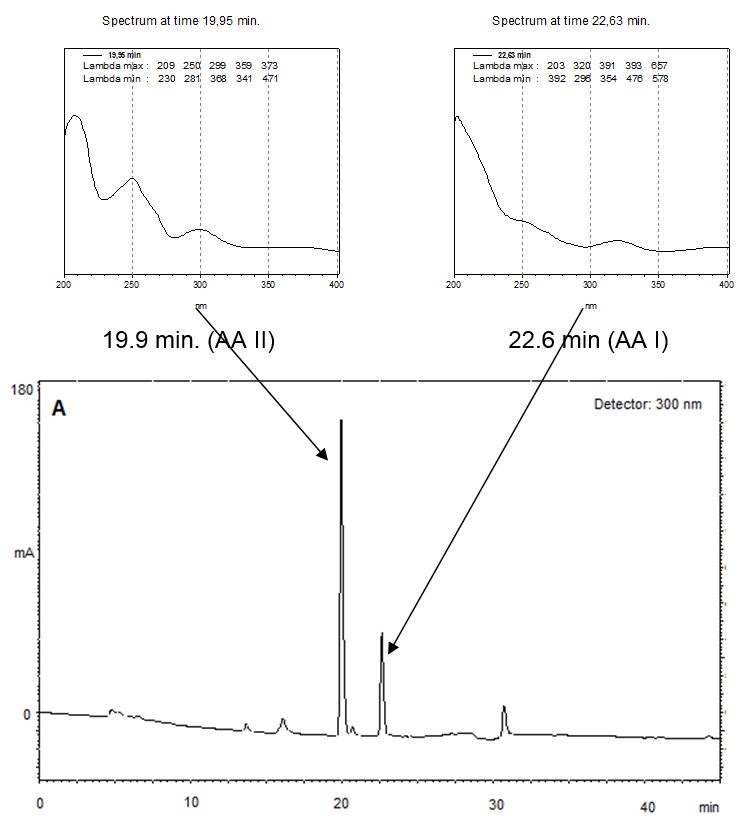
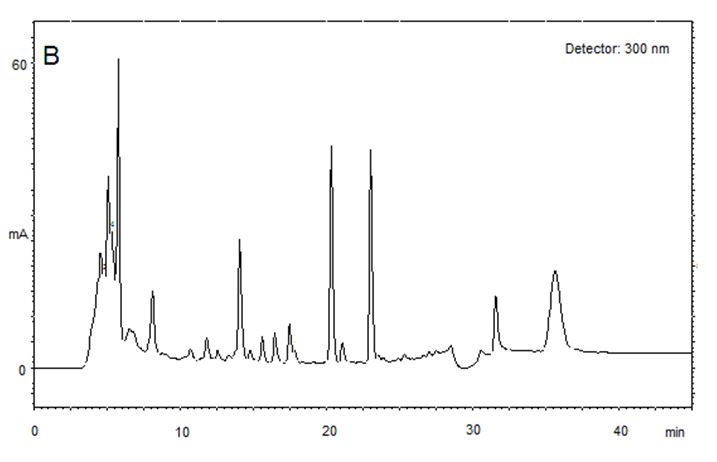
The quantitation results are summarized in TABLE 1, where it is seen the simultaneous occurrence of both aristolochic acids in all of them. The concentrations found ranged between 1.96 and 6.10 µg/mL for AAI and 2.22 and 11.55 µg/mL for AAII. In the present work we have set the UV detector to wavelengths 300 and 320 nm, respectively for AAII and AAI, instead of 224 and 254 nm as described by Schaneberg & Khan[14]. The choice was made to allow absorbance detection in peak maxima (see FIGURE 2).
TABLE 1: Amount of AAI and AAII present in samples evaluated.
| Sample | AAI | AAII | ||
| (%) | (µg/ml) | (%) | (µg/ml) | |
| 1 | 0.023 | 2,39 | 0.067 | 6,71 |
| 2 | 0.044 | 4,42 | 0.061 | 6,16 |
| 3 | 0.019 | 1,96 | 0.024 | 2,46 |
| 4 | 0.027 | 2,75 | 0.022 | 2,22 |
| 5 | 0.061 | 6,10 | 0.115 | 11,55 |
The quantitative analysis of the "milhomem" infusion samples collected in Aracaju, Brazil showed an expressive content of both AAI and AAII. These results suggest that a systematic assessment of the content of aristolochic acids has also to be done in the following species, all of them used as medicinal plants in Brazil (Mors et. al): Aristolochia birostris, A. cordigera, A. cymbifera, A. elegans, A. esperanzae, A. gigantea, A. triangularis, A. trilobata, among others. Screening these plants for the presence of AA is needed to evaluate whether their use pose any potential human health risk. Regarding the plants used to prepare the infusions the producers of the drink refer to the plants as "milhomem". Of the plants listed above, only A. birostris is not popularly referred to as "milhomem". All the others are known as "milhomem"[16].
Although regulatory agents, such as FDA in 2001, advised about the risk of using products suspected to contain AA's, several herb products are marketed without control. In Brazil, it is easy to access these plants in popular markets either as fresh plants or as in preparations such as the ones analyzed here. Many Aristolochia species are popularly recommended as medicines to treat snakebites, malaria, intestinal worms and so on[2,18-21].
Governmental agencies of different parts of world are banning products suspect to containing AA's. Several countries, such as UK, USA, Canada, Australia and New Zealand have suggested to reduce or prohibit the sale of herbal products containing aristolochic acids[13,22,23].
Conclusion
Aristolochic acids I and II were detected and quantified in samples of herbal preparations ("milhomem" sugar cane spirit infusions) collected in several popular markets in Aracaju, Sergipe, Brazil. In spite of the risks of consuming such preparations, the "milhomem" sugar cane spirit infusions has been used as a very popular drink, maybe due to the lack of scientific information on its dangers. The results found in the present work highlights the need that Brazilian public health authorities issue a warning on health risks involved in such practice. Thus, an urgent communication of these results to the community is strongly recommended and necessary to ensure a safe use of Brazilian medicinal plants.
Acknowledgements
This work was supported by grants from CNPq, FAPERJ and CAPES.
Referências
1. Ferreira LAQ, Marques CA. Garrafadas: uma abordagem analítica. Rev Fitos. 2018; 12(3):243-262. ISSN 2446-4775. [CrossRef].
2. Ortencio WB. 1997. Medicina Popular do Centro-Oeste, 2nd ed. Editora Thesaurus, Brasília, Brazil. ISBN 8570620729.
3. Van Wyk B, Wink M. 2004. Medicinal Plants of the World: An Illustrated Scientific Guide to the Important Medicinal Plants and Their Uses. Timber Press, Portland, OR, USA. ISBN 0881926027.
4. Giorgetti M, Negri G, Rodrigues E. Brazilian plants with possible action on the central nervous system – a study of historical sources from the 16th to 19th century. J Ethnopharmacol. 2007; 109(2):338-347. ISSN 0378-8741. [CrossRef].
5. Kelly LM, González F. Phylogenetic relationships in Aristolochiaceae. Systematic Botany. 2003; 28(2):236-249. ISSN 0363-6445. [CrossRef].
6. Kumar V, Poonam P, Prasad AK, Parmar VS. Naturally occurring aristolactams, aristolochic acid and dioxoaporphines and their biological activities. Nat Prod Rep. 2003; 20:565-583. ISSN 0265-0568. [CrossRef].
7. Zhang H, Cifone MA, Murli H, Erexson GL, Mecchi MS, Lawlor TE. Application of simplified in vitro screening test to detect genotoxicity of aristolochic acid. Food Chem Toxicol. 2004; 42(12):2021-2028. ISSN 0278-6915. [CrossRef].
8. Peters G, Hedwall PR. Aristolochic Acid Intoxication: A New Type of Impairment of Urinary Concentrating Ability. Arch Int Pharmacodyn Ther. 1963; 145(1):334-55. ISSN 0301-4533.
9. Cui M, Liu Zhi-Hong, Qiu Q, Li L, Li Lei-Shi. Tumor induction in rats following exposure to short-term dose aristolochic acid I. Mutagenesis. 2005; 20(1):45-49. ISSN 0267-8357. [CrossRef].
10. Heinrich M, Chan J, Wanke S, Neinhius C, Simmonds MSJ. Local uses of Aristolochia species and content of nephropatic aristolochic acid 1 and 2 – a global assement based on bibliographic sources. J Ethnopharmacol. 2009; 125(1):108-144. ISSN 0378-8741. [CrossRef]
11. Kessler DA. Cancer and Herbs. N Engl J Med. 2000; 342(23):1742-1743. ISSN 0028-4793. [CrossRef].
12. Arlt N M, Stiborova M, Schmeiser H H. Aristolochic acid as a probable human cancer hazard in herbal medicines: a review. Mutagenesis. 2002; 17(4):265-277. ISSN 0267-8357. [CrossRef].
13. Food and Drug Administration [www.fda.gov]. FDA concerned about botanical products, including dietary supplements, containing aristolochic acid. Access in: Jan. 2012. Available in: [Link].
14. Schaneberg BT, Khan IA. Analysis of products suspected of containing Aristolochia or Asarum species. J Ethnopharmacol. 2004; 94(1-2):245-249. ISSN 0378-8741. [CrossRef].
15. Ioset JR, Raoelison GE, Hostettmann K. Detection of aristolochic acid in Chinese phytomedicines and dietary supplements used as slimming regimens. Food Chem Toxicol. 2003; 41(1):29-36. ISSN 0278-6915. [CrossRef].
16. Mors WB, Rizzini, CT, Pereira, NA. 2000. Medicinal Plants of Brazil. Reference Publications, Michigan. ISBN 0917256425.
17. Lorenzi H, Matos FJA 2002. Plantas medicinais do Brasil: nativas e exóticas. Inst Plant, Nova Odessa. ISBN 8586714283.
18. Wu TS, Damu AG, Su CR, Kuo PP. Terpenoids of Aristolochia and their biological activities. Nat Prod Rep. 2004; 21(5):594-624. ISSN 0265-0568. [CrossRef].
19. Cardoso DR, Frederiksen AM, Silva AA, Franco DW, Skibsted LH. Sugarcane spirit extract of oak and Brazilian woods: antioxidant capacity and activity. Eur Food Res Technol. 2008; 227(4):1109-1116. ISSN 1438-2377. [CrossRef].
20. Toledo CEM, Britta EA, Ceole LF, Silva ER, Mello JCP, Filho BPD et al. Antimicrobial and cytotoxic activities of medicinal plants of the Brazilian Cerrado, using Brazilian cachaça as extractor liquid. J Ethnopharmacol. 2011; 133(2):420-425. ISSN 0378-8741. [CrossRef].
21. Therapeutical Goods Administration. Aristolochia fact sheet. Available in: [Link]. Access in: January 2012.
22. Health Canada [http://www.hc-sc.gc.ca]. 2002. Warning not to consume Longdan and Lung Tan Xie Gan products. Available in: [Link]. Access in: January 2012.
23. New Zealand Medicines and Medical Devices Safety Authority [http:www.medsafe.gov.nz]. Herbal, traditional and complementary medicines. [Link]. Access in: January 2012.