Artigo de Pesquisa
A preliminary study of the cytotoxicity of the protein extract of "abajerú" commercialized in markets
Abstract
The use of plants and their products for medical treatment is a quite common procedure in Brazil, especially for treatment of diabetes. In fact, several plants can demonstrate hypoglycemic effects in vitro assays. However, the use for human treatment requires the knowledge of their toxicological properties. The aim of this study was to evaluate the effect of protein extracts of Chrysobalanus icaco collected from natural habitats and of Eugenia astringens acquired from the market in Rio de Janeiro on the viability and migration of fibroblasts. E. astringens has a similar morphology as C. icaco and it is sold as Chrysobalanus in a popular market. Being a different plant, E. astringens expresses different proteins, and its protein extract has proved to possess higher toxic properties than C. icaco does. Cytotoxicity assays indicated that, as the protein extract concentration increases, fibroblast viability decreases. Only the E. astringens extract displayed cytotoxicity at all concentrations, in addition to reduced fibroblast migration. The results obtained in this study demonstrates that it is necessary integrative policies for rational use of medicinal plants and their commercialization, since the current use of medicinal plants may be inadequate, and it is of great importance for Public Health.
- Keywords:
- Cytotoxicity.
- Protein extract.
- Hypoglycemic plant.
- Chrysobalanus icaco.
- Eugenia astringens.
Introduction
Several plants are widely used for medical purposes by the population, but this use is most often made from a lay indication, without knowing the risks of toxic effects. Besides, there is no guarantee of the provenance and proper storage of these supposedly "medicinal plants". It is clear that there is a lack of incentive and scientifically-based information to integrative and complementary practices and actions to promote the safe and rational use of medicinal plants, including information on how the species should be prepared and used by the population[1].
The leaf extract (tea) of the plant Chrysobalanus icaco L. (Chrysobalanaceae), a species of restinga popularly known as "abajerú", is used in folk medicine because it exerts biological activities, such as the decrease of blood sugar levels, being indicated for the treatment of diabetes, besides be diuretic and antioxidant[2]. Another plant, Eugenia astringens Cambess. (Myrtaceae) which is morphologically similar to C. icaco, also is known by the same popular name of "abajerú" and is commercialized as C icaco[1 ,3]. These two species may not possess the same therapeutic and toxicological properties, which are of concern to Public Health. The attribution of hypoglycemic activity to E. astringens may indicate a misconception since other species of Myrtaceae have hypoglycemic potential[3]. So, in order to clarify the toxicological aspects of the extract obtained from these 2 plants, a cytotoxic assay was performed.
For cytotoxicity studies in animal cells several techniques, using distinct cell types as a target, are available. Cytotoxicity means the determination of any toxic effecs at the cellular level, such as changes in membrane permeability, cell death or enzymatic inhibition resulted from exposure to a toxicant, in this case, the studied plants or plant products[4].
Cell viability can be evaluated by several methods, among which the one which involves the conversion of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to formazan by mitochondrial reactivation in active-living cells[5]. The MTT assay is a standard colorimetric assay that estimates the cytotoxic potential of the samples, in addition to measuring the cellular proliferation of drug agents. Cell viability is expressed as a percentage of live cells from the tested material, comparing with the percentage of cells of the cytotoxicity positive control[6].
Diabetic patients may develop different complications in the body. Hyperglycemia alters leukocyte function, especially chemotaxis and phagocytosis, increasing the risk of bleeding and impairing the inflammatory and healing processes. Wound healing is the process of repairing and regenerating the dermis and epidermis that accompanies the lesions[7,8]. To measure the migration/motility of cells, distinctive of the healing process, the Scratch Wound Healing Assay is proposed. In this method, a crack imitates a wound in a monolayer of confluent cells so that the cells at the edge gradually move towards the crack [9]. The evaluation of the healing activity of plant extracts is scarce at the cellular level. Fibroblast cultures have been proposed as a method for the investigation of wound healing activity, since these cells are the main source of extracellular connective tissue matrix and the migration of fibroblasts is considered vital for rapid and effective skin repair damaged[9].
The lack of data about the toxicity of the protein extract of these 2 plants commercialized as "abajerú" (C. icaco and E. astringens), protein extracts of C. icaco collected directly from its natural habitats and of E. astringens acquired from the market of Rio de Janeiro was performed using the viability and migration of fibroblasts assay.
Experimental
Plant sampling
Chrysobalanus icaco leaves were collected directly from its natural habitats, Praia Grande – Arraial do Cabo - RJ (PG; -22,9696606, -42,0302859), Restinga de Massambaba – RJ (RMA; -22,9337727, -42,4267012), Marechal Deodoro – AL (AL; -9,7823233, -35,852364), as shown in the map (FIGURE 1). Eugenia astringens leaves were purchased on the market Mercadão de Madureira located in the North zone of Rio de Janeiro city.
Protein extraction
About 10 mg of each lyophilized sample were weighed into microtubes in triplicate. Samples were incubated in the presence of 400 μl lysis buffer (4% SDS 0.1 M Tris-HCl buffer pH 7.6) at 95°C for 15 min in a thermomixer. The lysate extract was frozen at -80°C for further quantification of total proteins by the Lowry method[10], using bovine serum albumin (2.0 mg/mL) as the standard for the analytical curve. Samples (2 μL) and analytical curve (0, 10, 20, 30, 40, 50, 60 and 70 μg/mL) were read in a Jasco V-530 spectrophotometer at the wavelength of 750 nm.
Cytotoxicity evaluation of protein extracts
This assay was performed as follows:
Cell culture
Fibroblasts (3T3 cell line) were kept in medium Dulbecco's Modified Eagle Medium (DMEM), containing 10% fetal bovine serum, L-glutamine (2 mM) and gentamicin (40 μg/mL) in incubator at 37°C and 5% CO2.
Cell viability assay
The effect of the protein extracts of Eugenia astringens (Mercadão de Madureira) and Chrysobalanus icaco (Restinga de Massambaba - RJ, Marechal Deodoro - AL, Praia Grande - RJ) on fibroblasts viability was evaluated through the MTT assay[11].
The cells were seeded in a 96 well plates and placed in CO2 incubator overnight. The cells were then treated with different sample solutions (1, 5, 10 and 20 μg/mL) in four replicates. The control group was treated only with the medium (DMEM). After treatment, a solution of MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (5 mg/mL in phosphate buffered saline - 1X PBS pH 7.4) was added to each well and incubated for 4 hours. Subsequently, the supernatant was discarded and 150 μl of dimethyl sulfoxide were added for solubilization of the formazan crystals. The absorbance was measured using a microplate spectrophotometer (DTX 880 Multimode Detector, Beckman Coulter), adjusted to 595 nm, and the optical density was calculated (EQUATION 1).
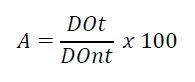
DOnt – optical density of non-treated cells
Scratch wound healing assay
The effect of the protein extracts of Eugenia astringens (Mercadão de Madureira), Chrysobalanus icaco (Restinga de Massambaba - RJ, Marechal Deodoro - AL, Praia Grande - RJ - branch) on fibroblast migration was evaluated through cell migration technique, method described by Liang et al.[7].
Cells (7 x 104 cells / well, measured by the Newbauer's chamber) were seeded in 24-well plates and maintained overnight for cell adhesion and formation of a monolayer at approximately 80% confluency. A small part of the monolayer was removed in the middle of the plate with a 200 μL pipette tip (a scratch is placed on the monolayer and the part removed is discarded). Cells were washed with phosphate buffered saline and treated with 5 μg/mL of the samples or culture medium (control) in triplicate. This exposure concentration at which some effects started to be observed in the cell viability assay was chosen to perform the present assay. Cell migration was assessed by photomicrographs at 0- and 24-hours post-exposure using an inverted microscope (Olympus IX70) with digital camera to measure the area of wound closure. The photomicrographs were analyzed using Image J software and cell migration was expressed as the area in pixels, so that the percentage of closure of the initial area formed was determined quantitatively (EQUATION 2).
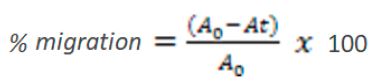
At – area after the scratch (time = 24 h)
Statistical analysis
The results of the cell viability and migration tests were expressed as mean ± standard error, performed in triplicate and analyzed statistically using analysis of variance (ANOVA), followed by Newman-Keuls post-test. The results were considered significant when p <0.05. All results were analyzed using GraphPad Prism® software version 5.01 (GraphPad Software Inc, San Diego CA, USA).
Results and Discussion
Plant identification and protein concentration
The identification of the studied plants was performed by a botanist from the Jardim Botânico do Rio de Janeiro. The plant purchased on the market (Mercadão de Madureira) was identified as Eugenia astringens Cambess., of family Myrtaceae, and the plants collected in Marechal Deodoro, Massambaba and Praia Grande as Chrysobalanus icaco L., plant from family Chrysobalanaceae.
Chrysobalanaceae can be morphologically diffeenciate from the Brazilian Myrtaceae species, by some characteristics, such as phylotaxia, which is alternating (and opposite in Myrtaceae). Nevertheless, the similar form of the leaves of C. icaco and E. astringens can cause confusion (FIGURE 2a, 2b, 2c), the E. astringens leaf has a fold around it facing the abaxial part (FIGURE 2c) not found in C. icaco.

This misconception has been previously reported[1,3], claiming that the trade of medicinal plants is not a safe source of sale, as it may be difficult for both the trader and the consumer to correctly identify a desirable plant. And yet there is the problem that different plants known by the same popular name are commercialized without proof of their pharmacological properties and toxicological safety[1], besides the adulteration possibilities. Unfortunately, in Brazil, the supervision of trade of medicinal plants by regulatory agencies is still incipient.
Total protein concentrations ranged from 30.18 to 54.95 μg μL-1 in Eugenia astringens, from 28.01 to 43.88 μg μL-1 in Chrysobalanus icaco.
Cytotoxicity evaluation of protein extracts
Fibroblasts (3T3 cell line) were submitted to the cell viability assay, exposed to different concentrations of protein extract and to the cell migration assay, exposed to a determined concentration of this extract.
Cell viability assay
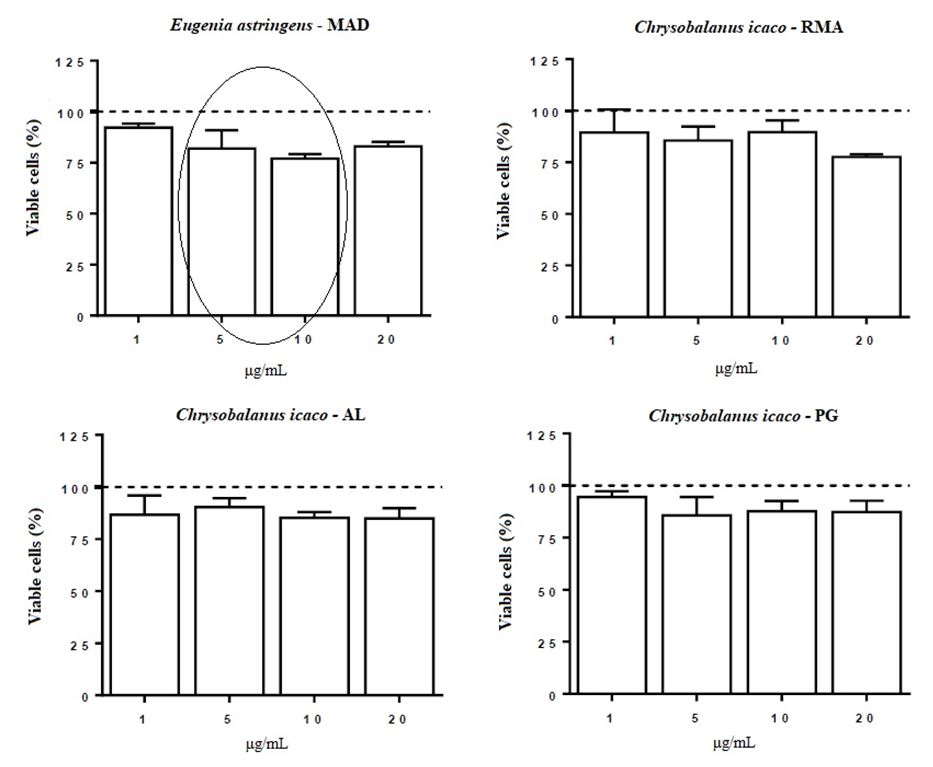
To evaluate the effects of extracts of E. astringens (MAD), C. icaco (RMA), C. icaco (AL) and C. icaco (PG) on fibroblast viability, the MTT assay was performed.
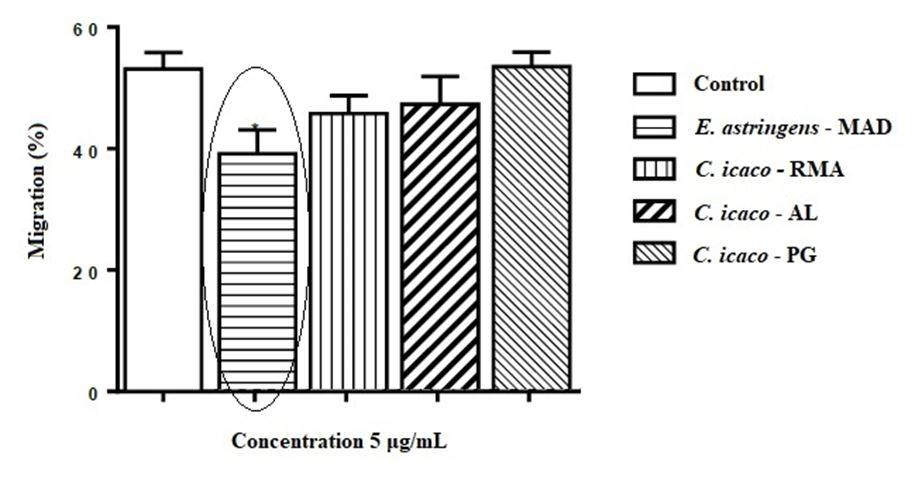
The results for the cell viability assay are shown in FIGURE 3, in which it can be observed and compared the reduction of fibroblasts viability among the species and protein extract concentration.
Treatment with E. astringens (MAD) at all concentrations tested, reduced cell viability, decreasing by 8.8% (1 μg/mL), 19.2% (5 μg/mL), 23% (10 μg/mL) and 17% (20 μg/mL) the percentage of viable cells. Exposure with C. icaco (RMA) at concentrations of 1, 5 and 10 μg/mL did not significantly alter the fibroblasts viability. On the other hand, the increase in concentration resulted in a decrease in the percentage of viable cells, leading to a reduction of 22.4% (P <0.001) in cell viability when the highest concentration (20 μg/mL) was used. Treatment with C. icaco (AL), in turn, induced a decrease in cell viability (8-16 %) at all concentrations tested, when compared to the control group. In addition, treatment with C. icaco (PG) at 1 μg/mL did not alter the viability of fibroblasts, whereas treatment with the other concentrations induced a decrease in cell viability (12-14%).
Scratch wound healing assay
To evaluate the effects of extracts of E. astringens (MAD), C. icaco (RMA), C. icaco (AL) and C. icaco (PG) on fibroblast migration, the cell migration assay (Scratch Wound Healing Assay) was performed.
As shown in FIGURE 4, treatment with C. icaco (RMA), C. icaco (AL) and C. icaco (PG) was not able to alter the migration rate of fibroblasts. On the other hand, it can be noted that the treatment with E. astringens led to a significant reduction in the migration of these cells by 26.04% (p <0.05), comparing to the control.
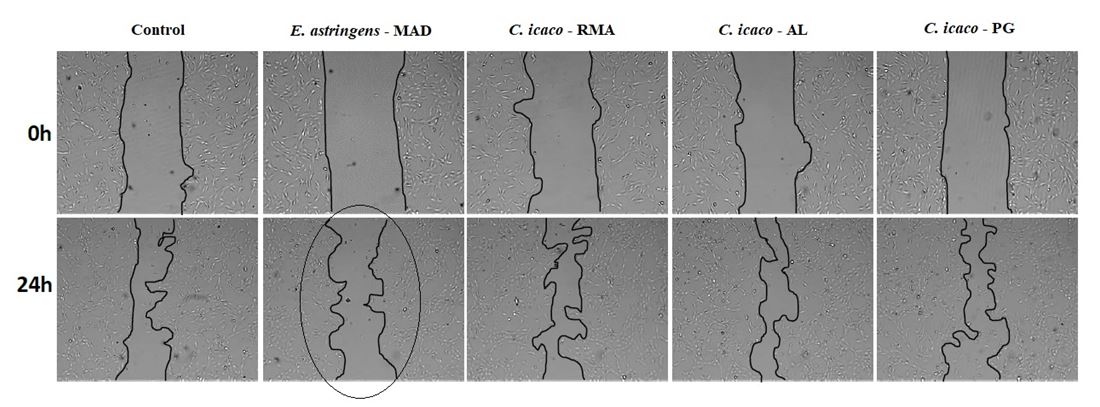
The migration of fibroblast is illustrated in FIGURE 5, in which can be observed a lower migration of these cells when treated with E. astringens protein extract than the C. icaco treatments, slowing wound closure.
There is a misunderstanding regarding the sale of "abajerú" in Mercadão de Madureira, where Eugenia astringens, of the same popular name, is sold in place of Chrysobalanus icaco. This is of great concern to Public Health, because C. icaco is popularly used as medicinal plant for treating diabetes, due to its hypoglycemic potential. Meanwhile the population consumes tea from the leaves of E. astringens, coming from these markets instead of C. icaco. Medicinal plants are widely used due to their easy accessibility, but they usually not have their efficacy and safety well established[1]. This fact can become a risk to those who use them since they can cause more deleterious effects than bring health benefits. It is of prime importance to inspect qualified individuals, traders, distributors and producers for regularization of the sale of medicinal plants.
Silva et al.[3] raised three hypotheses regarding the introduction of E. astringens, replacing C. icaco in popular marketing. First, it would be a strategy of the merchants to circumvent the competent oversight, by having the same popular name, but neither could it distinguish. A second hypothesis would be related to the difficulty in the recognition of the species by the collectors and sellers, as well as the consumers, due to the similar morphology. The last hypothesis would be the attribution of hypoglycemic activity to E. astringens by herbivores, since other species of Myrtaceae, such as pitanga (Eugenia uniflora L.), jambo (Eugenia jambos L.) and Eucalyptus, are used by the population for this purpose and have antioxidant, antifungal and antibacterial properties [12]. Also, the natural environments to which C. icaco occurs are restinga-type vegetation sites, which are usually areas of environmental protection, which makes it difficult to collect specimens of this species. Therefore, this could also be a hypothesis regarding the introduction of E. astringens, replacing C. icaco in popular marketing. This species is not hypoglycemic like C. icaco, which can lead to intoxication in those people who buy erroneously, thinking that they are acquiring the correct "abajerú" plant.
Since medicinal plants may also have unknown toxicological properties, the evaluation of toxicity, through in vitro tests, is required. Cytotoxicity of the extracts of medicinal plants, including those that are hypoglycemic, can affect cellular processes like healing that is crucial for diabetic patients. Hyperglycemia alters leukocyte function, increasing the risk of bleeding and impairing inflammatory and healing processes[13,14]. This difficulty in healing occurs due to cardiovascular complications, which cause blockage or decrease of blood circulation, and due to excess glucose, which can impair the functioning of the immune system. That is, diseased vessels decrease blood flow, especially to the legs and feet, harming the healing process and high glycemic levels incapacitate the body's defense cells[15].
Zandi et al.[5] verified the viability of fibroblasts (ovine line) in extracts of different plants (Aloe vera, hena, camomile, licorice, myrtle, mint, cinnamon, ginger and cedar) and that at the minimum concentration (6.25 μg/mL), the viability of dermal fibroblasts by MTT assay increased significantly in cedar (p <0.05). Combination of Aloe vera, mint extract and licorice significantly increased the viability of dermal fibroblasts (p <0.05). Aloe vera, which is also known for its hypoglycemic activity, has the ability to stimulate proliferation of L929 fibroblasts[9]. Calloni et al.[16] tested the phenolic extract of Plinia trunciflora from the same family as E. astringens on human lung fibroblast cells in the presence and absence of amiodarone, a drug used to treat arrhythmia, but which causes toxicity in the lungs. The extract rich in polyphenols was able to prevent the decrease of cellular viability (MTT test) and the ATP biosynthesis.
There are no studies testing the viability of fibroblasts exposed to protein extracts of Chrysobalanus icaco, but ethanolic extracts of these species prove to be important in cellular processes. Silva et al.[17] evaluated the antifungal activity of the C. icaco ethanolic extract, noting the inhibition of growth of Candida albicans and C. parapsilosis, strains exposed to this extract.
Pitz et al.[8] evaluated the in vitro activity of ethanolic extract of Plinia peruviana bark, the same family as the E. astringens, in healing processes and antioxidant activity in urinary fibroblasts (L929 cell line). The cell migration assay (Scratch Wound Healing Assay) indicated that none of the tested shell concentrations (0.5, 5, 25, 50 and 100 μg/ml) was able to increase the migration rate after 12 hours of incubation. These results demonstrate a positive effect of the peel on the wound healing process in the L929 fibroblast cell line, probably due to the antioxidant activity exhibited by phytochemicals in the extract. Manoj et al.[9] verified the effect of germplasm of Aloe vera, which is also hypoglycemic in L929 fibroblasts, through the cell migration assay, confirming the increase in fibroblast migration, which is important for regeneration and skin repair in case of injury.
There are no studies testing the viability and migration of fibroblasts exposed to protein extracts of C. icaco and E. astringes, however ethanolic extracts are used in studies to test toxicity of Eugenia species. The in vitro antioxidant activity of the ethanolic extract of Eugenia uniflora was determined by the inhibition of spontaneous autoxidation in brain homogenate, with the LD50 of 5.93 g/kg in mice[18]. In the phytotoxicity test of the Eugenia catharinae extract, it was observed that ethyl acetate and hexane fractions inhibited seed germination, while the hexane fraction showed higher inhibition of lettuce seedlings. E. catharinae demonstrated a considerable toxic activity, encouraging the search for the compounds responsible for this activity[19].
Conclusion
The assays to evaluate the toxicity of the protein extracts of the plants studied served to make aware of the sale and use of the Eugenia astringens plant, sold in place of Chrysobalanus icaco, since it reduced cell viability at all concentrations of the extract and decreased the fibroblast migration rate. These results showed that E. astringens can cause cytotoxic effects if consumed in larger doses.
The present work demonstrated the importance of research in the area of Public Health and the dissemination and communication to society of the results of scientific works since, due to the confounding of the use of medicinal plants, diabetic patients may opt for natural products in therapeutic use for the treatment of diabetes, in the wrong way.
Acknowledgements
The authors are thankful to CAPES and to Drª Viviane Kruel from the Research Institute of Botanical Garden of Rio de Janeiro for the identification of the specimens collected. The cytotoxicity assays were performed at the Laboratory of Cell Biology of the Federal University of Alagoas under the supervision of Dr. Emiliano Barreto. This study was carried out with financial support from the Coordination for the Improvement of Higher Education Personnel – Capes (Brasília, DF - Brazil), in the form of the first author's PhD grant (PROEX - 0487).
References
1. Bochner R, Fiszon JT, Assis MA, Avelar KES. Problems associated with the use of medicinal plants commercialized in "Mercadão de Madureira", Rio de Janeiro City, Brazil. Rev Bras Pl Med. 2012; 14(3): 537-47. [CrossRef].
2. Venancio VP, Almeida MR, Maria L, Antunes G. Cocoplum (Chrysobalanus icaco L.) decreases doxorubicin-induced DNA damage and downregulates Gadd45a, Il-1 β, and Tnf- α in vivo. Food Res Int. 2018; 105: 996-1002. [CrossRef].
3. Silva IM, Peixoto AL. O abajurú (Chrysobalanus icaco L. e Eugenia rotundifolia Casar.) comercializado na cidade do Rio de Janeiro, Brasil. Braz J Pharmacogn. 2009; 19(1 B): 325-32. [CrossRef].
4. Stockert JC, Blázquez-Castro A, Cañete M, Horobin RW, Villanueva Á. MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem. 2012; 114(8): 785-96. [CrossRef].
5. Zandi M, Masoumian M, Shariatinia A, Sanjabi MR. Optimal concentrations and synergistic effects of some herbal extracts on viability of dermal fibroblasts. Gene, Cell Tissue [Internet]. 2016; 3(4): Available from: [Link].
6. Stockert JC, Horobin RW, Colombo LL, Blázquez-Castro A. Tetrazolium salts and formazan products in Cell Biology: Viability assessment, fluorescence imaging, and labeling perspectives. Acta Histochem. 2018; 120(3): 159–67. [CrossRef].
7. Liang CC, Park AY, Guan JL. in vitro scratch assay: A convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007; 2(2): 329–33. [CrossRef].
8. Pitz HDS, Pereira A, Blasius MB, Voytena APL, Affonso RCL, Fanan S et al. In vitro evaluation of the antioxidant activity and wound healing properties of jaboticaba (Plinia peruviana) fruit peel hydroalcoholic extract. Oxid Med Cell Longev. 2016; 2016. [CrossRef].
9. Manoj K, Mishra D, Maity TK, Gupta SD. Screening wound-healing potential of different Aloe vera L. germplasms at the cellular level. Med Aromat Plant Sci Biotechnol. 2009; 3(1):62-4. Print ISSN 1752-3389.
10. Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1): 265-275. Print ISSN 1083-351X.
11. Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65(1-2): 55-63. [CrossRef].
12. Queiroz JMG, Suzuki MCM, Motta APR, Nogueira JMR, Carvalho EM. Aspectos populares e científicos do uso de espécies de Eugenia como fitoterápico. Rev Fitos. 2015; 9(2): 87-100. [CrossRef].
13. Negri G. Diabetes melito: plantas e princípios ativos naturais hipoglicemiantes. Rev Bras Ciên Farm. 2005; 41(2): 121-42. [CrossRef].
14. Aquino JA, Baldoni AO, Di Lorenzo OC, Cardoso CS, de Figueiredo RC, Sanches C. Pharmacotherapeutic empowerment and its effectiveness in glycemic control in patients with Diabetes Mellitus. Diabetes Metab Syndr Clin Res Rev. 2019; 13(1): 137-42. [CrossRef].
15. Hu F, Stampfer M, Haffner S, Solomon C, Willett W, Manson J. Elevated risk of cardiovascular disease prior to clinical diagnosis of Type 2 diabetes. Diabetes Care. 2002; 25(7): 1129–34. [CrossRef].
16. Calloni C, Silva Santos LF, Martínez LS, Salvador M. Data on cell viability of human lung fibroblasts treated with polyphenols-rich extract from Plinia trunciflora (O. Berg) Kausel. Data Br. 2016; 6: 728-31. [CrossRef].
17. Silva JPB, Peres AR, Paixão T, Silva A, Baetas A, Barbosa W et al. Antifungal activity of hydroalcoholic extract of Chrysobalanus icaco against oral clinical isolates of Candida Species. Pharmacogn Res. 2017; 9(1): 96-100. [CrossRef].
18. Auricchio MT, Bugno A, Barros SBM, Bacchi EM. Atividades antimicrobiana e antioxidante e toxicidade de Eugenia uniflora. Lat Am J Pharm. 2007; 26(1): 76-81. ISSN 0326-2383.
19. Colla G, Brighente IMC. Potencial tóxico dos extratos de Eugenia catharinae. In: 51º Congresso Brasileiro de Química. Meio Ambiente e Energia. São Luís, MA. 2011; 7(136). Acesso em: 27 jul. 2019. Disponível em: [Link] .