Artigo de Pesquisa
In vitro antileishmanial activity and phytochemical analysis of Casearia javitensis Kunth (Salicaceae)
Abstract
The objective of this study was to carry out phytochemical analysis of the bark extract of Casearia javitensis and to evaluate its antipromastigote activity against Leishmania amazonensis. The ethanol extract (EE) was fractionated in open-column chromatography to obtain the hexane (FrHex), dichloromethane (FrDcm), ethyl acetate (FrAcOET) and methanol (FrMeOH) fractions. The EE and its fractions were analyzed by thin layer chromatography (TLC). FrDcm was analyzed in high performance liquid chromatography with diode array detection (HPLC-DAD). The antipromastigote assay of Leishmania amazonensis and the cytotoxicity test against the acute monocytic leukemia cell line (THP-1) were performed by the MTT colorimetric assay. The phytochemical profile in TLC detected terpenes in all samples. However, in the ultraviolet spectrum (HPLC-DAD) for FrDcm suggested the presence of a phenolic compound. FrHex and FrDcm showed antipromastigote activity (IC50 = 116.6 ± 0.9 and 59.4 ± 1.1 μg mL-1, respectively) and low cytotoxicity (CC50 = 333.4 ± 3.2 and 241.2 ± 1.9 μg mL-1, respectively). The selectivity index for FrDcm was 4.1. We conclude that the FrDcm of C. javitensis has potantial as a leishmanicide, and this activity may be related to the presence of phenolic compound.
- Keywords:
- Leismaniasis.
- Antipromastigote.
- Cytotoxicity (THP-1).
- Phenolic Compound.
Introduction
The leishmaniases are a group of infectious disease, non-contagious, caused by protozoan parasites of the genus Leishmania, which affects skin and mucous membranes, transmitted to humans by the bite of infected female phlebotomine sandflies[1]. Endemic in 97 countries, an estimated 1 billion people live in an area at risk for the disease worldwide and there are no optimistic forecasts for its control for the next few years[1,2].
The Amazon was responsible for 57.3% of all cases of leishmaniasis in Brazil, with the states of Pará (30.2%), Mato Grosso (17.3%), Amazonas (15.0%) and Maranhão (13.4%) presented the most cases of Leishmaniasis in this region[3,4].
The treatment of leishmaniasis is mainly carried out with pentavalent antimonial drugs (sodium stibogluconate and meglumine antimoniate), whereas Pentamidine and Amphotericin B are the second choice in therapy[5]. However, the use of these drugs is questionable because of the limitations of toxicity, variable efficacy, requirements for parenteral administration and/or length of treatment regimens, and a high cost[5-7]. In addition, there are reports of parasite resistance, including the global HIV/AIDS epidemic with its accompanying impact on the immune system[8], having high Leishmania-HIV coinfection rates reported from Brazil, Ethiopia and the state of Bihar in India[9].
Moreover, in the Amazon, therapeutic failure may be related to the resistance of the parasite to drugs, to the irregular and distant use of individuals who live far enough away from health services[10]. Therefore, it is necessary to search for new therapeutic alternatives, with medicinal plants being an important source of bioactive compounds.
The Amazon region still presents high rates of infectious and parasitic diseases, with an expressiveness of deaths[11] that may be associated with the population's poor access to health services. Given this scenario, traditional knowledge about the use of medicinal plants often symbolizes the only therapeutic resource for these communities[12]. Among the plants, it stands out the genus Casearia (Salicaceae), composed of about 180 species throughout the tropical and subtropical regions of the globe, including Africa, Asia, Australia, North America and South America, and the Pacific islands[13], with 70 species belonging to the American continent and 37 present in Brazil[14], they stand out because they have species known for medicinal use in the Amazon, with potential cicatrizant activity[15].
The morphology of this genus is described as shrubs or small trees, found more frequently in forest vegetation[16], and are noted for their medicinal importance, with wide recommendations in traditional medicine for the treatment of wounds, ulcer[17], to cicatrizing[18], in addition to being used frequently for the treatment of different skin diseases[19], which may be related to leishmaniasis.
In addition, species of the Casearia genus present in the literature a study with antiparasitic activity, with activity between trypomastigotes of Trypanosoma cruzi (IC50 ranging from 0.53 to 2.77 μg mL-1) for clerodane diterpenes (casearins A, B, G, and J) and for Leishmania infantum promastigotes were also susceptible to casearins obtained from Casearia sylvestris, with IC50 values ranging from 4.45 to 9.48 μg mL-1[20], and the tricine compound, a flavone, obtained from of C. arborea demonstrated activity against the intracellular amastigotes of L. infantum, with an IC50 value of 56 µM[21].
Phytochemical studies on different species of the Casearia genus revealed the predominance of terpenoids, emphasizing clerodanic diterpenes[22]. Sesquiterpenes and monoterpenes, phenylpropanoids, steroids, phenolic glycosides, alkaloids and flavonoids have also been isolated[13,23]. Several of these chemical compounds, isolated from plant extracts, have been reported leishmanicidal activity[24], especially the terpene compounds obtained from Casearia Sylvestris[20] and flavonic compounds obtained from C. arborea[21] present in the genus Casearia.
The Casearia genus has 180 species, of which 287 compounds have already been isolated, and the terpenoids, in particular the diterpenoids clerodans, are the predominant and representative constituents in the Casearia genus[13]. Casearia javitensis Kunth is a species found very frequently in the Amazon region[15], Preliminary phytochemical studies detected the presence of terpenes and hexanoic and caproic acids[13,25], besides phenolic glycosides, steroids and flavonoids[23]. In analysis by gas chromatography–mass spectrometry (GC–MS) of the fractions obtained by fractionation of the dichloromethane extract, made it possible to detect triterpene friedelina and β-friedelanol and steroid β-sitosterol[15], but only in one study, 4-hydroxyphenyl-6-caffeoyl-β-L-glucoside and b-sitosterol were isolated from the hexane and methanol extracts of the leaves[26] (FIGURE 1).
However, several species of the genus lack validation studies of leishmanicidal activity, especially Casearia javitensis Kunth, with limited validation studies of biological activity and cytotoxicity assays.
In summary, despite the existence of phytochemical studies of the species, there are no reports of studies that evaluated the leishmanicidal activity. In view of the above, this work had the objective to evaluate the leishmanicidal potential of Casearia javitensis.
Material and methods
Plant material
The trunk bark of a C. javitensis individual was collected in an area of the Museu Paraense Emílio Goeldi in January 2016, in the municipality of Belém, state of Pará, Brazil Brazil (01°27'00'' S, 48°26'47'' W). The exsicata was deposited in the herbarium "João Murça Pires" of the respective museum under registration MG-Etn-00559.
Biological material
The parasite used was Leishmania (L.) amazonensis, isolated from a human case from Ulianópolis, Pará State (MHOM/BR/2009/M26361). Cell lines from acute monocytic leukemia (THP-1; ATCC Nº TIB 202) were purchased from the cell bank of Rio de Janeiro (BCRJ).
Phytochemical analysis
The powder from the barks underwent maceration with ethanol (1:10), and the macerated material was concentrated in a rotary evaporator until residue precipitation. The ethanol extract (EE, 0.125 g) was fractionated in an open chromatographic column, using as a stationary phase silica gel (0.1-0.2 mm/70-130 mesh) and mobile phase gradient solvents: hexane, dichloromethane, ethyl acetate and methanol.
In order to detect the presence of alkaloids and terpenes, EE, hexane fraction (FrHex), dichloromethane fraction (FrDcm), ethyl acetate fraction (FrAcOET) and methanol fraction (FrMeOH) (95:5:0.2) for alkaloids and Hexane, Ethyl Acetate (8:2), for the detection of terpenes the ultraviolet light (365 nm) and reagents from Dragendorff, and Liebermann-Burchard were used as developers[27]. FrDcm was subjected to high-performance liquid chromatography with a diode-array detector (HPLC-DAD).
Biological activity
Promastigotes of L. amazonensis were obtained after primary isolation in NNN (Novy–MacNeal–Nicolle) medium. Then the strains were subcultured and adapted to the RPMI (Roswell Park Memorial Institute) medium. The parasite was cultured at 26°C in RPMI 1640 medium supplemented with 10% heat inactivated fetal bovine serum (Gibco®, Grand Island, NY, USA), penicillin (100 U mL-1) and streptomycin (100 μg mL)[28].
The culture of log phase promastigotes was adjusted to 5x106 parasites 0.1 mL-1. Susceptibility test determination was performed on 96-well plates. The extract and fractions were analyzed in triplicate in a concentration gradient (200 to 3.125 μg mL-1). The negative control was performed only with parasites and the incubation medium. The positive control was made with amphotericin B (25 to 0.3906 μg mL). After 24 h of incubation at 26°C under 5 %CO2 atmosphere, 10 μl of tetrazolium salt (5 μg mL) was added to each well, and the parasites were quantified in an enzyme-linked immunosorbent assay reader[28]. The half-maximal inhibitory concentration (IC50) was determined by linear regression (Graph Pad Prism versão 5.04).
To determine cell viability, the colorimetric MTT (tetrazolium dye, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) metabolic activity assay was used[29]. THP-1 cells (4x105 cells 0.1 mL-1) were grown in RPMI-1640 medium (Sigma Aldrich®, EUA), supplemented with 5% fetal serum, maintained under 5% CO2 at 37°C. Cells were treated with extract and fractions at different concentrations (between 25 and 500 μg mL-1). After 24 h additional incubation MTT (5.0 mg mL-1) was added. The plate was incubated at 37°C in a 5% CO2 atmosphere for 4 h. Dimethylsulfoxide was added to each well to solubilize the formazan crystals. Optical density was determined at 490 nm (Stat Fax 2100 microplate reader, Awareness Technology, Inc., USA). The cytotoxic concentration (CC50) was determined by linear regression[29].
To determine the selectivity index (SI), the ratio of the CC50 value of the cytotoxic activity to the IC50 value of the antiprotozoal activity was calculated. When the SI value is >1, hat compound presents more active against protozoa and less active against mammalian cells[30].
Results and Discussion
The yield was of 3.67% (11.025 g) of EE. Fractionation of referred extract on an open chromatographic column generated fractions of different polarities, such as higher yield for methanol fraction (FrMeOH 91.95% - 7.356 g; TABLE 1).
| Samples | Yield (%) | TLC | |||
|---|---|---|---|---|---|
| Dragendorff | UV | Liebermann-Burchard | UV | ||
| EE | 3.67 | - | - | + | + |
| FrHex | 1.68 | - | - | + | + |
| FrDcm | 1.33 | - | - | + | + |
| FrAcOET | 4.06 | - | - | + | + |
| FrMeOH | 91.95 | - | - | + | + |
| Legend: TLC, thin-layer chromatography; UV, ultraviolet rays; EE, Ethanol Extract; FrHex, Hexane Fraction; FrDcm, Dichloromethane Fraction; FrAcOET, Ethyl Acetate Fraction; FrMeOH, Methanol Fraction. | |||||
The solvents influenced the final extraction content, the methanol solvent being the most selective for the extraction of the metabolites present in the casings Casearia javitensis and previous studies with Casearia sylvestris Sw. observed the presence of flavonic glycosides, with predominance of condensed tannins, metabolism groups[31].
Phytochemical studies on different species of the Casearia revealed the predominance of terpenoids, emphasizing clerodan diterpenes[22]. Therefore, this may explain the suggestive results for the presence of terpenes, and absence of alkaloids (TABLE 1). In the study of Casearia sylvestris Sw. also no alkaloid was observed[31].
Several of these chemical compounds, isolated from plant extracts, have proven leishmanicidal activity, such as terpenoids, aminoglycosteroids, aminosteroids, naphthoquinones, chalcones, iridioid glycosides, flavonoids, lignans and alkaloids[24].
Phytochemical studies of species of the genus, especially C. corymbosa, C. grewiifolia, C. membranacea and C. sylvestris allowed the isolation and structural characterization of 152 clerodan diterpenes, of which 41 were isolated from C. sylvestris[13]. More than 287 compounds have been identified from the Casearia genus and it can be said that terpenoids are the predominant class of metabolites, highlighting clerodane diterpenes[13]. For example, two clerodane diterpenes, Casearlucine A and Caseamembrol A also showed activity against L. amazonensis amastigote axenic stages (5.98 ± 6.8 and 10.5 ± 0.4 µM, respectively) and promastigote (11.1 ± 0.2 µM, for both)[32].
Phytochemical studies suggest the presence of terpenes in the EE and in all fractions obtained (TABLE 1). On the other hand, none of the samples presented a positive result for alkaloids. Following the thin layer chromatography profile analysis, the FrDcm fraction was selected for HPLC-DAD analysis, as it showed a better chromatographic profile when tested with the Liebermann-Burchard reagent and observed in ultraviolet light (UV). The FrDcm submitted to the analysis in HPLC-DAD presented a signal with greater intensity (TR = 46.422 min.) with UV spectrum with λ of 258.3 and 383.4 (FIGURE 2).
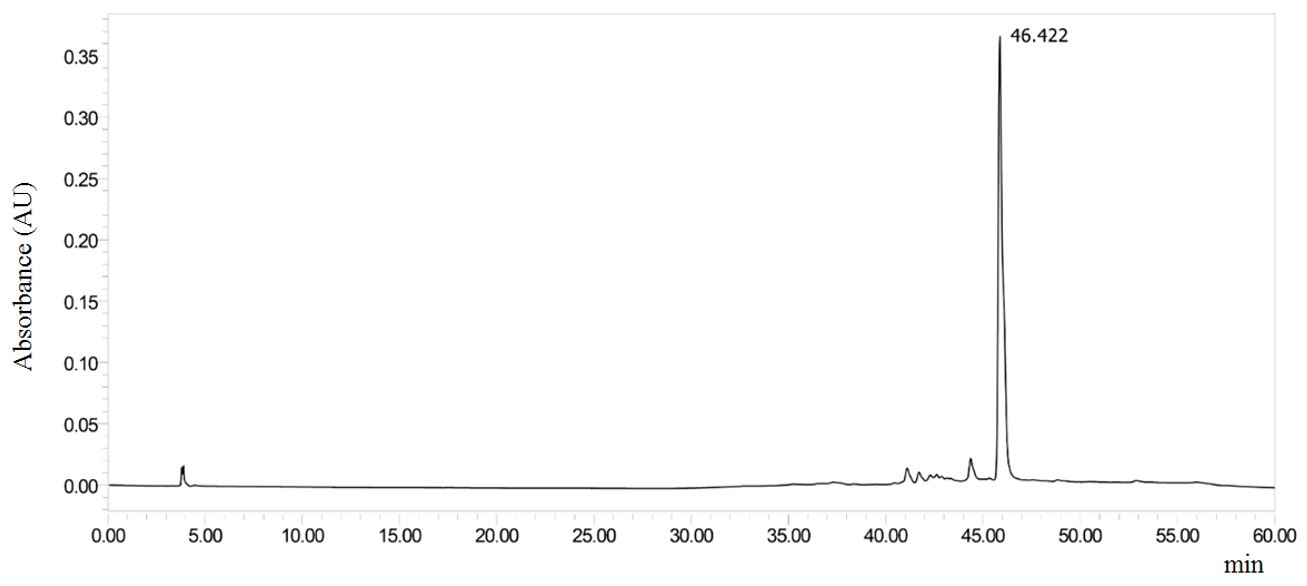
The chromatogram suggests that the major peak of FrDcm is a phenolic compound, since the absorption at 258.3 nm is suggestive of that of the band II (ring A, benzoyl portion). While the peak at 383.4 nm may be related to the Band I (ring B, cinamoyl portion) of the flavonoid[33,34]. From the maximum absorption value of Band I, it is possible to infer about the nature of the flavonoid, especially between flavones (304-350 nm) and flavonols (352-385 nm)[33,35]. The maxima absorption obtained from the FrDcm suggests that this compound is a flavonol.
Casearia javitensis extract was inactive against promastigote forms of L. amazonensis, presenting an CI50> 200 μg mL-1. FrHex (CI50 116.6 ± 0.9 μg mL-1) was considered moderately active. FrDcm (IC50 59.38 μg mL-1) obtained the best result among the samples tested, being considered active. FrAcOET (CI50> 200 μg mL-1) and FrMeOH (CI50> 200 μg mL-1) were inactive (TABLE 2). Thus, the results of the antipromastigote activity showed that the fractionation process contributed to the improvement of the biological activity, with a medium polarity profile (FrDcm) presenting better biological response.
| Samples | L. amazonensis CI50 (μg mL-1) | THP-1 cell line CC50 (μg mL-1) | SI |
|---|---|---|---|
| EE | >200 | 88.77 ± 2.8 | Nd |
| FrHex | 116.6 ± 0.9 | 333.4 ± 3.2 | 2.85 |
| FrDcm | 59.38 ± 1.1 | 241.2 ± 1.9 | 4.06 |
| FrAcOET | >200 | 30.5 ± 5.3 | Nd |
| FrMeOH | >200 | 101.4 ± 3.1 | Nd |
| Amphotericin B | 0.1699 ±0.0 | 162.8 ± 3.7 | 958.2 |
| Legend: THP-1, Human monocytic leukemia cell line; Nd, Not determined; SI, selective index; EE, Ethanol Extract; FrHex, Hexane Fraction; FrDcm, Dichloromethane Fraction; FrAcOET, Ethyl Acetate Fraction; FrMeOH, Methanol Fraction. | |||
In the cell viability assay, EE (CC50 88.77 μg mL-1), FrAcOET (CC50 30.5 μg mL-1) and FrMeOH (CC50 101.4 μg mL-1) showed toxicity to THP-1 monocytic cells. FrHex and FrDcm showed low cytotoxicity at the concentrations tested (CC50> 200 μg mL-1; TABLE 2). Thus, selective index results (TABLE 3) show that FrHex and FrDcm are 2.8 and 4.06 fold less toxic to THP-1 cells than to promastigote forms. Thus, it can be observed that fractions of low and medium polarity have secondary metabolites with low capacity to cause damage to the cell.
The activity observed in FrDcm may be related to the presence of flavonoids detected in HPLC-DAD. Two important members of the flavonoid family, namely quercetin and luteolin, have been reported to have marked leishmanicidal activity. Studies have shown that quercetin may induce the death of L. amazonensis by increasing the production of reactive oxygen species and by collapsing the mitochondrial potential[36]. Quercetin has also been reported to have multiple targets, including arginase, which is an important enzyme in the polyamine biosynthesis pathway[37], topoisomerase II in kinetoplasts, which induces DNA cleavage leading to apoptosis[38] and iron metal, which is important for parasite growth and replication[39].
It is worth mentioning that the observed values were obtained from fractions, in this way, new studies are required using the bioguided fractionation to aid in the isolation of the substances responsible for leishmanicide activity, which may improve by the inhibitory capacity of the parasite and consequently its selectivity .
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) – Finance Code 001. Furthermore, the authors express their gratitude to Laboratory of phytochemistry and microbiology of the Centro Universitário do Estado Pará (CESUPA) and Laboratory of Pharmacology and Neglected Diseases of Universidade Federal do Pará (UFPA).
References
1. World Health Organization (WHO). Leishmaniasis: Situation and trends. Disponível em: [Link]. Acesso em: 03 abr. 2019.
2. World Health Organization (WHO). Leishmaniasis. Disponível em: [Link]. Acesso em: 03 abr. 2019.
3. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Casos de Leishmaniose Tegumentar Americana. Brasil, grandes regiões e Unidades Federadas 1990 a 2017. Brasília: MS/SVS. Disponível em: [Link]. Acesso em: 30 mar. 2019.
4. Brasil. Ministério da Saúde. Secretaria de Vigilância em Saúde. Casos confirmados de Leishmaniose Visceral, Brasil, Grandes Regiões e Unidades Federadas 1990 a 2017. Brasília: MS/SVS. Disponível em: [Link]. Acesso em: 30 mar. 2019.
5. Rath S, Trivelin LA, Imbrunito TR, Tomazela DM, Jesus MN, Marzal PC et al. Antimoniais empregados no tratamento da leishmaniose: estado da arte. Quim Nova. 2003; 26(4): 550-5. ISSN 1678-7064. [CrossRef].
6. Croft SL, Coombs GH. Leishmaniasis- current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003; 19(11): 502-8. ISSN 1471-4922. [CrossRef] [PubMed].
7. Oliveira RAG, Lima EO, Vieira WL, Freire KRL, Trajano VN, Lima IO et al. Estudo da interferência de óleos essenciais sobre a atividade de alguns antibióticos usados na clínica. Rev Bras Farmacogn. 2005; 16(1): 77-82. ISSN 0102-695X. [CrossRef].
8. Ponte-Sucre A, Gamarro F, Dujardin JC, Barrett MP, López-Vélez R, García-Hernández R et al. Drug resistance and treatment failure in leishmaniasis: A 21st century challenge. PLoS Negl Trop Dis. 2017; 11(12): e0006052. ISSN 1935-2727. [CrossRef] [PubMed].
9. World Health Organization (WHO). Leishmaniasis: Leishmania-HIV co-infection. Disponível em: [Link]. Acesso em: 03 abr. 2019.
10. Rodrigues AM, Hueb M, Santos TARR, Fontes CJF. Fatores associados ao insucesso do tratamento da leishmaniose cutânea com antimoniato de meglumina. Rev Soc Bras Med Trop. 2006; 39(2): 139-45. ISSN 1678-9849. [CrossRef].
11. Viana RL, Freitas CM, Giatti LL. Saúde ambiental e desenvolvimento na Amazônia legal: indicadores socioeconômicos, ambientais e sanitários, desafios e perspectivas. Saúde Soc. 2016; 25(1): 233-46. ISSN 1984-0470. [CrossRef].
12. Lira TM, Chaves MPSR. Comunidades ribeirinhas na Amazônia: organização sociocultural e política. Interações (Campo Grande). 2016; 17(1): 666-76. ISSN 1518-7012. [CrossRef] [Link].
13. Xia L, Guo Q, Tu P, Chai X. The genus casearia: a phytochemical and pharmacological overview. Phytochem Rev. 2015; 14(1): 99-135. ISSN1572-980X. [CrossRef].
14. Shen Y-C, Wang C-H, Cheng Y-B, Wang L-T, Guh J-H, Chien C-T et al. New cytotoxic clerodane diterpenoids from the leaves and twigs of Casearia membranacea. J Nat Prod. 2004;67(3): 316-21. ISSN 0163-3864. [CrossRef].
15. Wyrepkowski CDC. Estudo fitoquímico e bioatividade de extratos de Casearia javitensis Kunth. Manaus. 2010. 143 f. Dissertação de Mestrado [Programa de Pós-Graduação Multi-institucional em Biotecnologia] - Universidade Federal do Amazonas, UFAM. Manaus. 2010. [Link].
16. Marquete R. O gênero Casearia no estado do Rio de Janeiro, Brasil - Flacourtiaceae. Rio de Janeiro, 2005. 167 f. Dissertação de Mestrado [Programa de Pós-Graduação em Botânica] - Instituto de Pesquisas Jardim Botânico do Rio de Janeiro, IJBRJ. Rio de Janeiro, 2005. [Link].
17. Ferreira PMP, Costa-Lotufo LV, Moraes MO, Barros FWA, Martins AMA, Cavalheiro AJ et al.Folk uses and pharmacological properties of Casearia sylvestris: a medicinal review. An Acad Bras Ciênc. 2011; 83(3): 1373-84. ISSN 0001-3765. [CrossRef].
18. Souza CD, Felfili JM. Uso de plantas medicinais na região de Alto Paraíso de Goiás, GO, Brasil. Acta Bot Bras. 2006; 20(1): 135-42. ISSN 1677-941X. [CrossRef].
19. Rodrigues VEG, Carvalho DA. Levantamento etnobotânico de plantas medicinais no domínio do cerrado na região do alto rio grande - Minas Gerais. Ciên Agrotecn. 2001; 25(1). Disponível em: [Link]. Acesso em: 03 abr. 2019.
20. Bou DD, Tempone AG, Pinto EG, Lago JH, Sartorelli P. Antiparasitic activity and effect of casearins isolated from Casearia sylvestris on Leishmania and Trypanosoma cruzi plasma membrane. Phytomedicine. 2014; 21(5): 676-81. ISSN 0944-7113. [CrossRef] [PubMed].
21. Santos AL, Yamamoto ES, Passero LFD, Laurenti MD, Martins LF, Lima ML et al. Antileishmanial Activity and Immunomodulatory Effects of Tricin Isolated from Leaves of Casearia arborea (Salicaceae). Chem Biodivers. 2017; 14(5): e1600458.ISSN 1612-1880. [CrossRef] [PubMed].
22. Ferreira PMP, Santos AG, Tininis AG, Costa PM, Cavalheiro AJ, Bolzani VS et al. Casearin X exhibits cytotoxic effects in leukemia cells triggered by apoptosis. Chem Biol Interact. 2010; 188(3): 497-504. ISSN 0009-2797. [CrossRef] [Link].
23. Rayanil K, Nimnoun C, Tuntiwachwuttikul P. New phenolics from the wood of Casearia grewiifolia. Phytochem Letters.2012;5(1): 59-62. ISSN 1874-3900. [CrossRef].
24. Bezerra J, Costa GC, Lopes TC, Carvalho ICDS, Patrício FJ, Sousa SM et al. Avaliação da atividade leishmanicida in vitro de plantas medicinais. Rev Bras Farmacogn. 2006; 16(supl.): 631-37. ISSN 0102-695X. [CrossRef].
25. Prieto AM, Dos Santos AG, Oliveira APS, Cavalheiro AJ, Silva DHS, Bolzani VS et al. Assessment of the chemopreventive effect of casearin B, a clerodane diterpene extracted from Casearia sylvestris (Salicaceae). Food Chem Toxicol. 2013; 53(1): 153-59. ISSN 0278-6915. [CrossRef] [PubMed].
26. Achin-Espinar MT, Souza MCS, Nunez CV. Isolamento de 4-hidroxifenil-6-cafeoil-β-L-glicosídeo e β-sitosterol e avaliação das atividades antibacteriana, antioxidante e tóxica sobre Artemia salina de Casearia javitensis. Rev Fitos. 2017; 10(3): 268-282. ISSN 2446-4775. [CrossRef] [Link].
27. Wagner H, Bladt S, Zgainski EM. Plant Drug Analysis: A Thin Layer Chromatography. Berlin: Springer-Verlag. 1984. ISBN: 978-3-642-00573-2.
28. Mota EF, Rosário DM, Veiga ASS, Brasil DS, Silveira FT, Dolabela MF. Biological activities of Croton palanostigma Klotzsch. Pharmacogn Mag. 2015; 11(43): 601-6. ISSN 0976-4062. [CrossRef] [PubMed].
29. Mosmann T. Rapid colorimetric assay for cellular grouth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983; 65(1-2): 55-63. ISSN 0022-1759. [CrossRef] [PubMed].
30. Almeida TL, Monteiro JÁ, Lopes GKP, Chiavelli LUR, Santin SMO, Silva CC et al. Estudo químico e atividades antiproliferativa, tripanocida e leishmanicida de Maxillaria picta. Quím Nova2014; 37(7): 1151-7. ISSN 0100-4042. [CrossRef].
31. Luz SFB, Sato MEO, Duarte MR, Santos CAM. Parâmetros para o controle da qualidade de folhas de Casearia sylvestris SW. - Guaçatonga.Rev Bras Farmacogn.1998; 7-8(1): 1-11. ISSN 0102-695X. [CrossRef].
32. Jullian V, Bonduelle C, Valentin A, Acebey L, Duigou A-G, Prévost M-F et al. New clerodane diterpenoids from Laetia procera (Poepp.) Eichler (Flacourtiaceae), with antiplasmodial and antileishmanial activities. Bioorg Med Chem Lett. 2005; 15(22): 5065-70. ISSN 0960-894X. [CrossRef] [PubMed].
33. Mabry TJ, Markham KR, Thomas MB. The ultraviolet spectra of flavones and flavonols. Berlin: Springer; 1970. p. 41-164. ISBN: 978-3-642-88458-0.
34. Alonso-Salces RM, Barranco A, Abad B, Berrueta LA, Gallo B, Vicente F. Polyphenolic profiles of Basque cider apple cultivars and their technological properties. J Agric Food Chem. 2004. 52(10): 2938-52. ISSN 1520-5118. [CrossRef].
35. Markham KR, Mabry TJ. Ultraviolet-visible and proton magnetic resonance spectroscopy of flavonoids. In: Harborne JB, Mabry TJ, Mabry H, editors. The Flavonoids. London: Chapmann and Hall; 1975. p. 62-75. ISBN: 978-1-4899-2909-9.
36. Fonseca-Silva F, Inácio JDF, Canto-Cavalheiro MM, Almeida-Amaral EE. Reactive oxygen species production and mitochondrial dysfunction contribute to quercetin induced death in Leishmania amazonensis. PLoS ONE 2011; 6(2): e14666. [CrossRef] [PubMed].
37. Da Silva ER, Maquiaveli CC, Magalhães PP. The leishmanicidal flavonols quercetin and quercitrin target Leishmania (Leishmania) amazonensis arginase. Exp Parasitol. 2012; 130(3): 183-8. ISSN 0014-4894. [CrossRef] [PubMed].
38. Mittra B, Saha A, Chowdhury AR, Pal C, Mandal S, Mukhopadhyay S et al. Luteolin, an abundant dietary component is a potent anti-leishmanial agent that acts by inducing topoisomerase II-mediated kinetoplast DNA cleavage leading to apoptosis. Mol Med. 2000; 6(6): 527-41. ISSN 1528-3658. [CrossRef].
39. Sen G, Mukhopadhyay S, Ray M, Biswas T. Quercetin interferes with iron metabolism in Leishmania donovani and targets ribonucleotide reductase to exert leishmanicidal activity. J Antimicrob Chemother. 2008; 61(5): 1066-75. ISSN 1460-2091. [CrossRef].